The global surgical suction devices market is experiencing steady expansion, driven by rising surgical volumes, increasing demand for minimally invasive procedures, and the growing emphasis on infection control in healthcare settings. According to Mordor Intelligence, the market was valued at approximately USD 3.2 billion in 2023 and is projected to grow at a CAGR of 6.8% from 2024 to 2029. Similarly, Grand View Research reports robust growth in the medical suction equipment sector, citing advancements in device design and rising adoption in emergency and critical care as key contributing factors. Within this landscape, the Yankauer suction device—a staple in surgical and emergency medicine—remains a critical tool for airway management and oral cavity evacuation. With increasing demand for high-quality, ergonomic, and disposable variants, a competitive manufacturing ecosystem has emerged. Below, we spotlight the top 8 Yankauer suction device manufacturers leading innovation, compliance, and global market penetration.
Top 8 Yankauer Suction Device Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Yankauer Suction
Domain Est. 2000
Website: accessdata.fda.gov
Key Highlights: Device Manufacturer’s Name: Medline Industries, Inc. Date of this Report … Per site reporter: Medline will follow-up with Medtronic (manufacture of Yankauer ……
#2 CONMED Suction Instruments and Tubing
Domain Est. 1995
Website: conmed.com
Key Highlights: Rigid Yankauer Suction Instruments. This sterile, single use device features large lumens to provide rapid aspiration and resist clogging….
#3 PhotonSaber Y
Domain Est. 1995
Website: stryker.com
Key Highlights: Handheld illuminator incorporated in a traditional Yankauer suction platform. This innovative device provides on-field illumination, aspiration, smoke ……
#4 Medical Suction Handles
Domain Est. 1996
Website: cardinalhealth.com
Key Highlights: Cardinal Health offers a complete line of rigid and flexible suction handles/sets, including Yankauer, Poole, Frazier and Sigmoid instruments….
#5 Suction Yankauers and Kits
Domain Est. 1997
Website: amsino.com
Key Highlights: AMSure Suction Yankauers are designed with a slip-resistant, lightweight handle that helps provide optimal control and maneuverability….
#6 SSCOR SDC Catheter
Domain Est. 1998
Website: sscor.com
Key Highlights: The SSCOR SDC Catheter has a larger inside diameter than a standard Yankauer suction tip to facilitate the removal of fluids as well as solid material. The ……
#7 Yankauer Suction Device
Domain Est. 2001
Website: gsource.com
Key Highlights: $18 delivery 90-day returnsgSource Yankauer Suction Device are crafted from high quality German or US surgical stainless steel. Explore our inventory of Yankauer Suction Device and…
#8 Yankauer Suction Tubes and Tips
Domain Est. 2003
Website: gcmedica.com
Key Highlights: The flexi-clear yankauer handle is mainly used for suction therapy in surgical operation, which belongs to the part of the surgical suction assembled….
Expert Sourcing Insights for Yankauer Suction Device
H2: Market Trends for Yankauer Suction Devices in 2026
The global market for Yankauer suction devices is expected to witness steady growth through 2026, driven by increasing demand for efficient airway management tools in surgical, emergency, and critical care settings. This analysis outlines key market trends anticipated to shape the Yankauer suction device landscape by 2026.
-
Rising Surgical and Emergency Procedures
The growing volume of surgical procedures—particularly in ambulatory surgery centers and emergency departments—is a primary driver of Yankauer suction device adoption. As minimally invasive surgeries and outpatient procedures increase, so does the need for reliable suction tools to manage blood, fluids, and debris during operations. The aging global population, which is more prone to chronic conditions requiring surgical intervention, further amplifies this trend. -
Expansion in Emerging Markets
Emerging economies in Asia-Pacific, Latin America, and Africa are expected to contribute significantly to market growth by 2026. Increased healthcare spending, infrastructure development, and government initiatives to improve access to medical devices are enhancing the penetration of essential surgical tools like the Yankauer suction device. Local manufacturing and distribution partnerships are also helping reduce costs and improve device availability. -
Shift Toward Disposable and Single-Use Devices
Infection control remains a top priority in healthcare settings, accelerating the shift from reusable to single-use Yankauer suction tips. Hospitals and clinics are increasingly adopting disposable variants to minimize cross-contamination risks and comply with sterilization regulations. This trend is supported by advancements in cost-effective, high-quality plastic manufacturing, making disposables both safe and economical. -
Innovation in Design and Materials
Manufacturers are focusing on ergonomic redesigns, improved suction efficiency, and biocompatible materials to enhance performance and user comfort. Devices with anti-clog features, better fluid handling, and enhanced grip are gaining traction. Additionally, transparent materials allow for real-time monitoring of aspirated content, improving clinical decision-making during procedures. -
Integration with Advanced Suction Systems
Future Yankauer devices are increasingly being designed to integrate seamlessly with wall-mounted and portable electric suction units, including smart suction systems that offer variable pressure controls and alarms. This integration supports precision in fluid management and reduces the risk of tissue damage, particularly in delicate procedures. -
Regulatory and Reimbursement Landscape
Stringent regulatory standards—especially from the FDA and EU MDR—are influencing product design and quality assurance processes. Compliance with these regulations is becoming a competitive advantage. On the reimbursement front, favorable coding for procedures involving suction use supports continued adoption in both inpatient and outpatient settings. -
Impact of Telehealth and Home Care Expansion
While primarily used in clinical environments, the growth of post-operative home care and telehealth monitoring is prompting interest in portable suction solutions. Although Yankauer devices are less common in home settings, adaptations for home-based tracheostomy or palliative care may open niche markets by 2026.
Conclusion
By 2026, the Yankauer suction device market is poised for moderate but consistent growth, supported by procedural volume increases, emphasis on infection control, and technological enhancements. Manufacturers that prioritize innovation, cost efficiency, and compliance with global standards will be best positioned to capitalize on evolving healthcare demands.

Common Pitfalls When Sourcing Yankauer Suction Devices (Quality, IP)
Sourcing Yankauer suction devices—especially from international suppliers—can present significant challenges related to product quality and intellectual property (IP). Being aware of these pitfalls is crucial for ensuring patient safety, regulatory compliance, and legal protection.
Poor Manufacturing Quality and Material Standards
One of the most prevalent issues is receiving Yankauer devices made from substandard materials or with inconsistent manufacturing practices. Low-quality plastics may become brittle, crack during use, or leach harmful chemicals. Poorly molded tips or shafts can compromise suction efficacy or pose breakage risks during procedures. Inconsistent dimensions may affect compatibility with standard suction tubing or wall outlets, leading to performance failures in critical situations.
Lack of Regulatory Compliance and Certification
Many sourced products, particularly from unverified suppliers, lack proper regulatory approvals such as FDA 510(k), CE marking, or compliance with ISO 13485 (quality management for medical devices). Without valid certifications, these devices cannot be legally marketed or used in clinical settings in many countries. Relying on falsified or outdated documentation exposes healthcare providers and distributors to significant legal and safety risks.
Inadequate Sterility and Packaging
Reusable Yankauer devices must withstand repeated sterilization without degradation, while single-use versions must arrive sterile and remain so until opened. Poor packaging—such as non-intact seals or non-sterile barrier materials—can compromise sterility, increasing the risk of healthcare-associated infections (HAIs). Suppliers may cut corners on packaging integrity to reduce costs, creating a serious patient safety hazard.
Counterfeit or IP-Infringing Products
A major intellectual property concern is the proliferation of counterfeit or copycat Yankauer devices that mimic the design of patented or trademarked products (e.g., the original Yankauer design or branded versions). These may infringe on design patents, utility patents, or trademarks, exposing the purchaser or distributor to legal liability. Using or selling such devices can lead to cease-and-desist orders, product seizures, or lawsuits from IP holders.
Ambiguous or Missing Documentation
Suppliers may fail to provide comprehensive technical documentation, including Instructions for Use (IFU), biocompatibility reports, or material disclosures. This lack of transparency hinders proper risk assessment, regulatory submissions, and traceability—especially critical in the event of a product recall or adverse event investigation.
Inconsistent Product Performance
Even if a sourced Yankauer device appears visually correct, performance inconsistencies—such as clogging, poor suction control, or ergonomic flaws—can affect clinical outcomes. Devices not rigorously tested under real-world conditions may fail during critical use, endangering patients and undermining trust in the supply chain.
Conclusion
To mitigate these risks, conduct thorough due diligence on suppliers, verify regulatory certifications, request product samples for testing, and consult legal counsel to assess IP risks. Prioritizing quality and compliance over cost savings ensures safer patient care and protects your organization from legal and reputational damage.
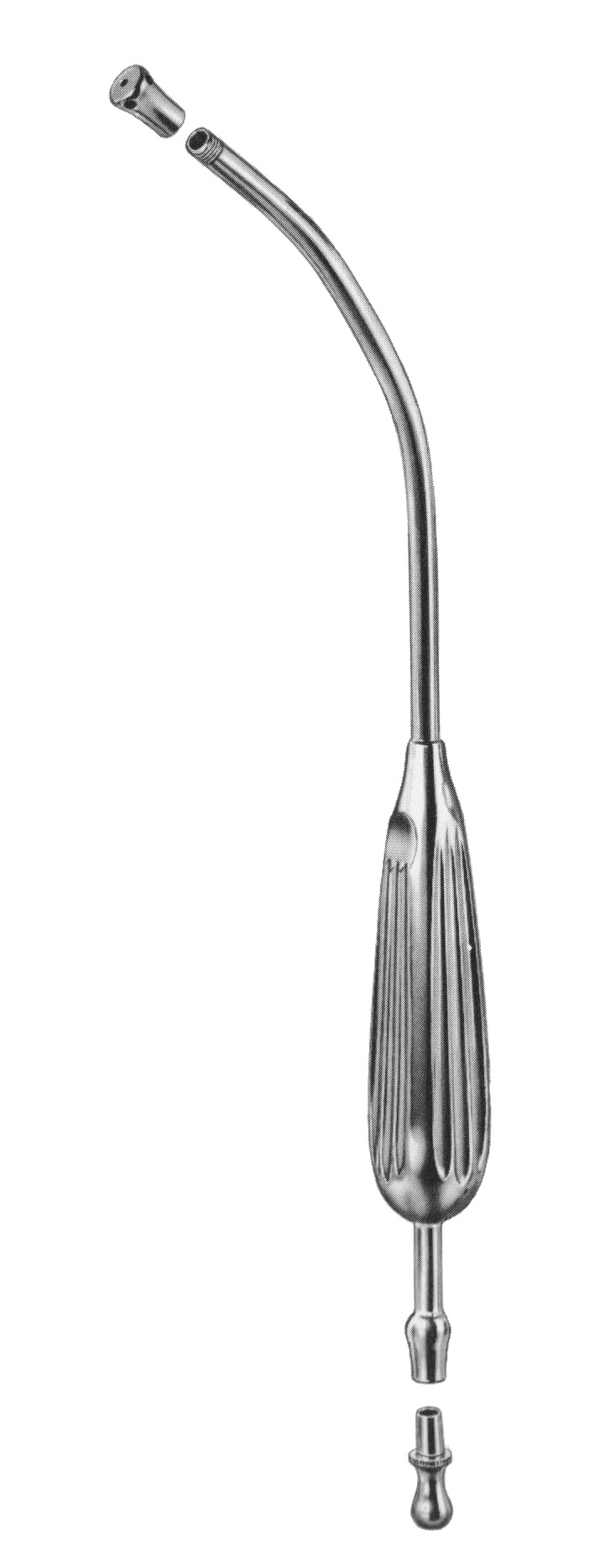
Logistics & Compliance Guide for Yankauer Suction Device
This guide outlines the essential logistics and regulatory compliance considerations for the distribution, handling, and use of Yankauer Suction Devices to ensure safety, efficacy, and adherence to global standards.
Regulatory Classification and Approval
The Yankauer Suction Device is typically classified as a medical device under international regulatory frameworks. In the United States, it is regulated by the FDA as a Class I or Class II medical device under 21 CFR 876.5875 (Suction Apparatus), often exempt from premarket notification (510(k)) unless modified. In the European Union, it falls under Regulation (EU) 2017/745 (MDR) and is generally classified as Class I (non-sterile) or Class Is (sterile). Manufacturers must ensure CE marking with proper technical documentation and a Quality Management System (QMS) certified to ISO 13485. Similar classifications apply in Canada (Health Canada Class I or II), Australia (TGA Class I or IIa), and other major markets.
Labeling and Packaging Requirements
All packaging must comply with local regulatory labeling standards. Minimum requirements include device name (“Yankauer Suction Tip” or equivalent), manufacturer name and address, model/lot number, expiration date (if applicable), sterility status (e.g., “Sterile, Single-Use Only”), and UDI (Unique Device Identification) in markets requiring it (e.g., USA, EU, China). Labels must include appropriate symbols per ISO 15223-1 (e.g., sterile, single-use, latex-free if applicable). Packaging must maintain sterility during transport and storage and be tamper-evident. Language must conform to local requirements (e.g., bilingual in Canada, French in France).
Storage and Handling Conditions
Yankauer Suction Devices must be stored in a clean, dry environment with controlled temperature (typically 5°C to 40°C or as specified by the manufacturer) and low humidity to prevent degradation. Devices should be protected from direct sunlight, radiation, and physical damage. Sterile products must remain sealed until point of use. Handling should follow good distribution practices (GDP) to prevent contamination or damage. Stock rotation (FIFO – First In, First Out) must be implemented to prevent use of expired products.
Transportation and Distribution
Transportation must maintain the integrity of packaging and sterility. Use validated shipping containers when necessary, especially for temperature-sensitive or sterile items. Shipments should be tracked using barcodes or RFID where applicable. Compliance with international shipping regulations (e.g., IATA for air freight, IMDG for sea) is required if combined with other regulated goods. Distributors must be authorized and compliant with local medical device distribution laws (e.g., EU MDR Authorized Representative, FDA Registered Distributor).
Import/Export Documentation
Export and import of Yankauer Suction Devices require accurate documentation, including commercial invoice, packing list, bill of lading/airway bill, Certificate of Conformity (e.g., CE, FDA listing), and Certificate of Free Sale (if requested). U.S. exports require filing through the Automated Export System (AES) if value exceeds $2,500 or license is required. Importers must ensure compliance with local customs and medical device registration (e.g., ANVISA in Brazil, CFDA/NMPA in China). Use Harmonized System (HS) codes correctly (e.g., 9018.90 for surgical instruments).
Post-Market Surveillance and Vigilance Reporting
Manufacturers and distributors are responsible for monitoring device performance post-market. Any adverse events or malfunctions (e.g., tip fracture, material failure) must be reported to relevant authorities per local timelines: FDA MedWatch (within 30 days), EU Vigilance System (within 15 days for serious incidents), and equivalent systems globally. Maintain a robust complaint handling system and conduct root cause analysis when necessary. Field Safety Notices (FSNs) or recalls must be issued if required.
Environmental and Disposal Compliance
Yankauer Suction Devices are single-use medical devices and must be disposed of as medical waste in accordance with local regulations (e.g., OSHA, CDC, EPA in the U.S., or EU Directive 2008/98/EC). Proper segregation, containment, and documentation of disposal are required. Facilities must follow biohazard waste protocols when contaminated with bodily fluids. Recycling is generally not permitted due to infection control standards.
Training and Staff Competency
Personnel involved in logistics, sales, and clinical use must receive appropriate training. Logistics staff should understand GDP, cold chain management (if applicable), and documentation requirements. Clinical users (e.g., nurses, physicians) must be trained on proper use, infection control, and disposal per manufacturer instructions and institutional policies. Training records should be maintained as part of quality compliance.
Audit and Quality Assurance
Regular internal and external audits must be conducted to ensure compliance with ISO 13485, FDA QSR, EU MDR, and other applicable standards. Audit scope should include suppliers, manufacturing, warehousing, distribution, and post-market processes. Corrective and preventive actions (CAPA) must be documented and implemented for any non-conformances identified.
Conclusion
Proper logistics and compliance management for Yankauer Suction Devices are critical to patient safety and regulatory adherence. Stakeholders must maintain rigorous control across the product lifecycle—from manufacturing through distribution to end-use and disposal—ensuring alignment with global and local medical device regulations.
Conclusion for Sourcing Yankauer Suction Devices
In conclusion, sourcing Yankauer suction devices requires a strategic approach that balances quality, cost, regulatory compliance, and supplier reliability. These essential airway management tools play a critical role in both surgical and emergency care settings, making it imperative to select products that meet clinical standards and user expectations. Key considerations include adherence to international safety and sterilization standards (such as ISO 13485 and FDA approval), material quality (e.g., medical-grade silicone or PVC), ergonomic design for ease of use, and compatibility with existing suction systems.
Evaluating potential suppliers based on track record, certification, production capacity, and after-sales support ensures a reliable supply chain. Additionally, bulk purchasing agreements and long-term contracts may offer cost efficiencies without compromising on product integrity. It is also advisable to conduct sample testing and on-site audits when possible to verify product consistency and manufacturing practices.
Ultimately, the successful sourcing of Yankauer suction devices supports improved patient outcomes, clinical efficiency, and infection control in healthcare environments. A well-informed procurement strategy, grounded in clinical needs and regulatory requirements, will ensure a sustainable and safe supply of this vital medical device.