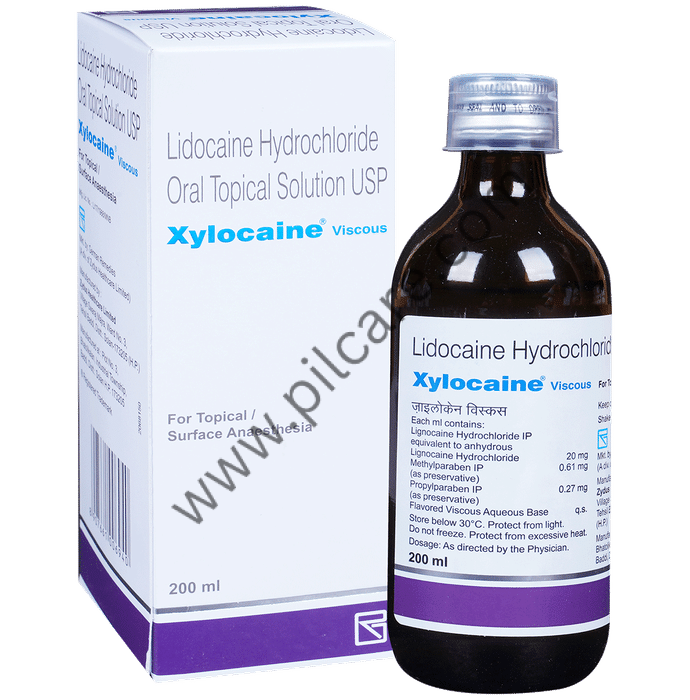
The global numbing agents market, which includes viscous lidocaine products such as Xylocaine Viscous, is witnessing steady expansion driven by rising demand for local anesthetics in dental, gastrointestinal, and ENT procedures. According to Grand View Research, the global local anesthetics market was valued at USD 2.1 billion in 2022 and is projected to grow at a CAGR of 4.7% from 2023 to 2030. This growth is fueled by increasing outpatient surgical procedures, a growing geriatric population, and expanded use in endoscopic and diagnostic applications. As demand for effective oromucosal anesthetics rises, manufacturers of Xylocaine Viscous—a trusted brand of viscous lidocaine hydrochloride—play a critical role in meeting clinical needs. The following list highlights the top five manufacturers based on market presence, regulatory compliance, production capacity, and product availability, reflecting key players shaping the competitive landscape in this expanding sector.
Top 5 Xylocaine Viscous Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 XYLOCAINE VISCOUS API Manufacturers
Domain Est. 2014
Website: pharmacompass.com
Key Highlights: View All Manufacturers & Suppliers of XYLOCAINE VISCOUS API with Drug Master Files (DMF), CEP/COS, Japanese DMFs, Written Confirmation (WC) details listed ……
#2 Xylocaine (Lidocaine)
Domain Est. 1995
Website: rxlist.com
Key Highlights: Xylocaine (lidocaine HCl) 2% Jelly is indicated for prevention and control of pain in procedures involving the male and female urethra….
#3 LIDOCAINE VISCOUS
Domain Est. 1997
Website: dailymed.nlm.nih.gov
Key Highlights: Lidocaine Viscous 2% (Lidocaine Hydrochloride Oral Topical Solution, USP) The 2% oral topical solution is supplied as a clear, colorless, viscous solution….
#4 Lidocaine Viscous
Domain Est. 1997
Website: veteranshealthlibrary.va.gov
Key Highlights: Lidocaine viscous, a local anesthetic, is used to treat the pain of a sore or irritated mouth and throat often associated with cancer chemotherapy and certain ……
#5 Product information
Domain Est. 2002
Website: health-products.canada.ca
Key Highlights: Product information ; Current status: Dormant ; Current status date: 2018-07-12 ; Original market date: · 1954-12-31 ; Product name: XYLOCAINE VISCOUS 2% ; DIN :….
Expert Sourcing Insights for Xylocaine Viscous
H2: 2026 Market Trends for Xylocaine Viscous
As the pharmaceutical landscape evolves toward personalized care and cost-efficient treatments, Xylocaine Viscous (viscous lidocaine 2%) is expected to experience moderate but stable market dynamics through 2026. Several key trends are shaping its trajectory, including shifting clinical applications, competitive generic pressures, regulatory considerations, and evolving patient needs.
-
Stable Demand in Palliative and Oncology Care
Xylocaine Viscous remains a standard-of-care topical anesthetic for managing oral and pharyngeal mucositis—particularly in cancer patients undergoing chemotherapy and radiation. With rising global cancer incidence and an aging population, demand in palliative and supportive care settings is projected to remain steady through 2026. Its off-label use for dysphagia-related pain and esophagitis also contributes to consistent clinical utility. -
Increased Generic Competition and Price Pressure
As a long-established medication with expired patents, Xylocaine Viscous faces robust generic competition. By 2026, multiple generic manufacturers are expected to maintain a saturated market, leading to downward pricing pressure. This enhances accessibility but limits revenue growth potential for branded versions. Market consolidation among generic pharmaceutical firms may further streamline distribution but reduce marketing incentives for older formulations. -
Regulatory and Safety Scrutiny
The FDA and other global regulatory bodies continue to monitor the safety of viscous lidocaine, particularly regarding systemic absorption and risks in pediatric and elderly populations. Black box warnings for methemoglobinemia remain in effect, prompting cautious prescribing. By 2026, increased emphasis on risk mitigation strategies—such as patient education and dosing protocols—may influence prescribing patterns, potentially slowing growth in outpatient settings. -
Shift Toward Alternative Formulations
Innovations in local anesthetics, including lidocaine in spray, gel, and transmucosal film formats, may erode Xylocaine Viscous’ market share. These newer formulations often offer improved dosing accuracy, reduced systemic absorption, and better patient compliance. By 2026, healthcare providers may increasingly favor these alternatives, especially in ambulatory and home-care environments. -
Supply Chain and Manufacturing Challenges
Global supply chain vulnerabilities—exacerbated by geopolitical tensions and regulatory inspections—could impact the availability of Xylocaine Viscous. Manufacturing delays or quality control issues at key facilities may lead to intermittent shortages, affecting market stability. Companies investing in resilient supply chains and alternative production sites are likely to gain competitive advantage. -
Regional Market Variations
In emerging markets, Xylocaine Viscous is expected to maintain stronger growth due to lower healthcare costs and limited access to newer alternatives. In contrast, mature markets like the U.S. and Western Europe may see declining use as providers adopt guideline-recommended alternatives. Regional regulatory approvals and reimbursement policies will play a critical role in shaping adoption rates.
Conclusion:
By 2026, Xylocaine Viscous is anticipated to hold a niche but essential role in symptom management, particularly in oncology and palliative care. While market growth will be constrained by generics, safety concerns, and newer formulations, its proven efficacy and low cost will sustain baseline demand. Strategic positioning through patient support programs, clinical education, and integration into treatment pathways will be vital for maintaining relevance in a competitive and evolving analgesic market.

Common Pitfalls When Sourcing Xylocaine Viscous (Quality, IP)
Sourcing Xylocaine Viscous (viscous lidocaine) requires careful attention to quality and intellectual property (IP) considerations to avoid regulatory, safety, and legal risks. Below are key pitfalls to watch for:
Poor Quality Control and Substandard Products
One of the most significant risks in sourcing Xylocaine Viscous is encountering substandard or counterfeit products. This is particularly common when sourcing from unverified suppliers or regions with weak regulatory oversight. Poor quality may include incorrect active pharmaceutical ingredient (API) concentration, contamination, improper viscosity, or use of non-pharmaceutical-grade excipients. Such defects can compromise patient safety and lead to ineffective treatment or adverse reactions.
Lack of Compliance with Pharmacopoeial Standards
Xylocaine Viscous must meet stringent quality standards such as those defined in the United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), or other relevant national pharmacopoeias. A common pitfall is sourcing products that do not comply with these standards—either due to inadequate testing, lack of documentation, or deliberate misrepresentation. Always verify that the product has valid Certificates of Analysis (CoA) and is manufactured in facilities compliant with Good Manufacturing Practices (GMP).
Inadequate Intellectual Property Due Diligence
Xylocaine is a registered trademark of Pfizer Inc. for its lidocaine hydrochloride oral topical solution. Sourcing generic viscous lidocaine under the name “Xylocaine Viscous” without proper IP clearance can lead to trademark infringement. Buyers must ensure suppliers have the legal right to use branding or labeling that references “Xylocaine” and confirm whether the product is an authorized generic or a legally marketed equivalent. Failure to conduct IP due diligence may result in shipment seizures, legal action, or reputational damage.
Unverified Supplier Credentials and Manufacturing Practices
Engaging with suppliers who lack transparent manufacturing histories or verifiable GMP certifications increases the risk of receiving poor-quality or non-compliant products. Some suppliers may operate through intermediaries or third-party platforms without direct access to manufacturing facilities, making quality assurance difficult. Always conduct on-site audits or request third-party audit reports (e.g., from FDA, EMA, or WHO) to confirm manufacturing standards.
Regulatory Non-Conformity in Target Markets
Different countries have varying regulatory requirements for topical anesthetics. A product approved in one jurisdiction may not meet the specifications or registration requirements of another. Sourcing without confirming alignment with the target market’s regulatory framework (e.g., FDA 510(k), EMA approval, or local health authority registration) can result in import denials or market withdrawal.
Inconsistent Batch-to-Batch Quality
Even with certified suppliers, inconsistent production processes can lead to variability in viscosity, pH, or drug release profile. This inconsistency undermines therapeutic reliability and can affect patient outcomes. Ensure suppliers have robust quality management systems and provide batch-specific testing data to confirm consistency.
Inadequate Packaging and Stability Data
Improper packaging (e.g., non-child-resistant containers or light-sensitive materials) can degrade the product. Additionally, sourcing without reviewing full stability data (shelf life under recommended storage conditions) risks distributing an unstable or ineffective product. Confirm that packaging complies with regulatory standards and that real-time stability studies support the claimed expiry date.
Avoiding these pitfalls requires due diligence in supplier qualification, regulatory compliance verification, and IP risk assessment—ensuring both patient safety and legal integrity in the supply chain.
Logistics & Compliance Guide for Xylocaine Viscous
Storage & Handling
Xylocaine Viscous (lidocaine hydrochloride) must be stored at controlled room temperature, typically between 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C to 30°C (59°F to 86°F) as per USP standards. Protect from light and excessive heat. Keep containers tightly closed to prevent contamination. Avoid freezing. Ensure storage areas are secure, dry, and inaccessible to unauthorized personnel, especially due to its pharmaceutical and controlled substance-like handling requirements in some jurisdictions.
Transportation Requirements
Transport Xylocaine Viscous in accordance with local and international pharmaceutical transport regulations (e.g., GDP – Good Distribution Practice). Use temperature-monitored vehicles when necessary to maintain the cold chain integrity. Packaging must be tamper-evident and designed to prevent breakage or leakage during transit. Shipments should be labeled clearly with product name, concentration, lot number, expiration date, and handling instructions (e.g., “Protect from Light,” “Do Not Freeze”). Ensure transport documentation includes safety data sheets (SDS) and complies with DOT (Department of Transportation) or equivalent regulatory body guidelines for medicinal products.
Regulatory Compliance
Xylocaine Viscous is an FDA-approved prescription medication (NDC: 0002-3025-11, 2% w/v) and must be distributed and handled in compliance with the U.S. Food, Drug, and Cosmetic Act. Prescribers and dispensers must adhere to state and federal prescribing laws. While lidocaine is not classified as a controlled substance under the Controlled Substances Act (CSA), its sale and distribution are restricted to licensed healthcare professionals and pharmacies. Maintain accurate records of inventory, dispensing, and distribution as required by state pharmacy boards and FDA regulations. International shipments must comply with destination country import regulations, including necessary permits, labeling in local language, and adherence to ICH guidelines.
Documentation & Recordkeeping
Maintain complete and accurate records for a minimum of 2 years (or as required by local law), including batch-specific certificates of analysis (CoA), shipping logs, temperature monitoring data, and chain of custody documentation. All adverse event reports related to product quality or safety must be documented and reported to the manufacturer (AstraZeneca) and regulatory authorities as required by FDA MedWatch (Form 3500A). Ensure labeling complies with 21 CFR Part 201, including adequate directions for use, warnings, and safety information.
Safety & Disposal
Personnel handling Xylocaine Viscous should wear appropriate personal protective equipment (PPE) such as gloves and eye protection to avoid accidental exposure. In case of spillage, follow facility SOPs using absorbent materials and dispose of waste in accordance with local hazardous waste regulations. Expired or unused product must be disposed of via authorized pharmaceutical waste disposal companies in compliance with EPA and DEA (if applicable) guidelines. Never dispose of in household trash or sewage systems.
Import/Export Considerations
For international movement, verify that Xylocaine Viscous is authorized for use in the destination country. Obtain required import licenses and ensure packaging and labeling meet local regulatory standards. Documentation must include a commercial invoice, bill of lading, certificate of pharmaceutical product (CPP), and any additional forms mandated by customs or health authorities. Coordinate with a licensed freight forwarder experienced in pharmaceutical logistics to ensure compliance with IATA and WHO GDP standards.
In conclusion, sourcing Xylocaine Viscous requires careful consideration of regulatory compliance, supplier credibility, and proper documentation to ensure patient safety and adherence to healthcare standards. It is essential to obtain the product through licensed pharmaceutical distributors or reputable suppliers that meet FDA or equivalent regulatory body requirements. Verifying the authenticity, expiration date, and storage conditions of the medication upon receipt is crucial in maintaining its efficacy. Additionally, healthcare providers should follow appropriate procurement protocols, including prescription validation and secure handling, to prevent misuse. Ultimately, responsible sourcing of Xylocaine Viscous supports safe clinical use and enhances patient care outcomes.