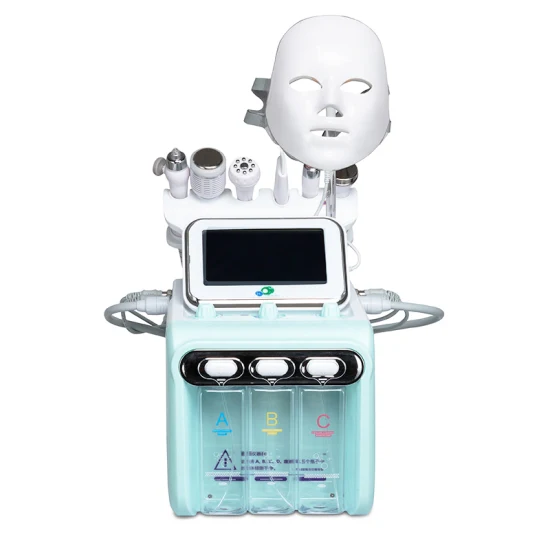
Sourcing Guide Contents
Industrial Clusters: Where to Source Wholesale China Microdermabrasion Machine

Professional B2B Sourcing Report 2026
SourcifyChina | Senior Sourcing Consultant
Deep-Dive Market Analysis: Sourcing Wholesale Microdermabrasion Machines from China
Executive Summary
The global demand for professional and at-home skincare devices continues to expand, driven by rising consumer awareness, aesthetic medicine growth, and the proliferation of beauty tech. Microdermabrasion machines—non-invasive exfoliation devices used in dermatology clinics, spas, and homecare—represent a high-growth segment within the aesthetic equipment market. China has emerged as the dominant manufacturing hub for these devices, offering competitive pricing, scalable production, and a mature supply chain ecosystem.
This report provides a strategic overview for global procurement managers seeking to source wholesale microdermabrasion machines from China. It identifies key industrial clusters, evaluates regional manufacturing strengths, and delivers a comparative analysis of major production provinces—Guangdong and Zhejiang—based on price, quality, and lead time.
Market Overview: China’s Role in Microdermabrasion Machine Manufacturing
China accounts for over 70% of global production of aesthetic and skincare devices, including microdermabrasion systems. The country benefits from:
- Mature electronics and precision engineering capabilities
- Established supply chains for medical-grade plastics, motors, vacuum pumps, and digital interfaces
- Strong OEM/ODM infrastructure tailored to international regulatory standards (CE, FDA, ISO 13485)
- Competitive labor and logistics costs
Most manufacturers specialize in crystal-based and diamond-tip microdermabrasion systems, with increasing innovation in RF-combined models and smart connectivity features (e.g., app integration, usage tracking).
Key Industrial Clusters for Microdermabrasion Machine Production
China’s manufacturing of microdermabrasion machines is concentrated in two primary industrial clusters:
1. Guangdong Province
- Core Cities: Guangzhou, Shenzhen, Dongguan
- Cluster Strengths:
- Center of China’s electronics and medical device OEM ecosystem
- High concentration of ISO 13485-certified manufacturers
- Proximity to Hong Kong for export logistics
- Strong R&D and design capabilities (especially in Shenzhen)
- Dominant in high-end and export-oriented production
2. Zhejiang Province
- Core Cities: Hangzhou, Ningbo, Wenzhou
- Cluster Strengths:
- Cost-efficient mass production
- Well-developed small-to-mid-sized enterprise (SME) network
- Specialization in mid-tier aesthetic devices
- Strong logistics via Ningbo-Zhoushan Port (world’s busiest by cargo tonnage)
- Competitive pricing with moderate quality control
Comparative Analysis: Guangdong vs Zhejiang
| Parameter | Guangdong | Zhejiang |
|---|---|---|
| Average Unit Price (FOB) | $85 – $180 (mid to high-end models) | $55 – $110 (standard models) |
| Quality Level | High; ISO 13485, CE, FDA-compliant options widely available; precision engineering; superior materials (medical-grade aluminum, ABS) | Moderate; suitable for general spa and home use; variable QC; fewer certified suppliers |
| Lead Time (MOQ 100–500 units) | 25–35 days (including QC and packaging) | 18–25 days (faster turnaround, less customization) |
| Customization Capability | High (ODM/OEM with UI, branding, packaging) | Medium (limited to basic modifications) |
| Export Readiness | Excellent (English-speaking teams, compliance documentation) | Good (documentation may require third-party verification) |
| Best For | Premium brands, medical distributors, regulated markets (EU, US) | Budget-focused buyers, emerging markets, private label resale |
Strategic Sourcing Recommendations
- For High-End or Medical-Grade Procurement:
- Partner with Guangdong-based manufacturers in Shenzhen or Guangzhou.
- Prioritize ISO 13485 and CE-certified suppliers for regulatory compliance.
-
Budget for higher unit costs but benefit from reliability and scalability.
-
For Cost-Optimized Bulk Orders:
- Consider Zhejiang suppliers for economical models targeting home-use or entry-level spas.
- Conduct on-site audits or third-party inspections to mitigate quality variance risks.
-
Leverage shorter lead times for faster inventory turnover.
-
Hybrid Sourcing Strategy:
-
Dual-source: Use Guangdong for flagship models and Zhejiang for volume-driven SKUs to balance cost and quality.
-
Compliance & Certification:
- Confirm that suppliers provide technical files, RoHS, and electrical safety reports (IEC 60601).
- Audit production facilities for cleanroom standards and traceability protocols.
Conclusion
China remains the most strategic source for wholesale microdermabrasion machines, with Guangdong leading in quality and innovation and Zhejiang offering cost efficiency and speed. Global procurement managers should align sourcing decisions with brand positioning, regulatory requirements, and target market expectations.
By leveraging regional strengths and implementing rigorous supplier qualification processes, buyers can achieve optimal balance across cost, quality, and supply chain resilience in 2026 and beyond.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
February 2026
Confidential – For B2B Procurement Use Only
Technical Specs & Compliance Guide

SourcifyChina Sourcing Report: Technical & Compliance Guidelines for Wholesale China Microdermabrasion Machines (2026)
Prepared for Global Procurement Managers
Date: January 15, 2026 | Report ID: SC-CH-MD-2026-01
Executive Summary
Sourcing microdermabrasion machines from China requires rigorous technical validation and regulatory due diligence. As Class I/II medical devices (region-dependent), these units demand precision engineering and adherence to global safety standards. This report details critical quality parameters, mandatory certifications, and defect mitigation strategies to minimize supply chain risk and ensure market compliance.
I. Technical Specifications & Quality Parameters
A. Core Material Requirements
| Component | Material Specification | Critical Tolerance | Verification Method |
|---|---|---|---|
| Vacuum Pump Housing | Medical-Grade 316L Stainless Steel (ASTM F138) | ±0.02mm | Material Cert. + Spectrography |
| Handpiece Body | Medical-Grade Polycarbonate (ISO 10993-1 Biocompatible) | ±0.05mm | RoHS/REACH Testing |
| Abrasive Crystals | Aluminum Oxide (Al₂O₃), 50-150µm, Sterile (ISO 13485) | ±5µm particle size | Laser Diffraction Analysis |
| Vacuum Tubing | Silicone (USP Class VI), Non-PVC, Oil-Free | Wall thickness: ±0.1mm | Pressure Decay Test |
B. Performance Tolerances
- Vacuum Pressure Range: 0–15 inHg (±0.5 inHg accuracy at 10 inHg)
- Crystal Flow Rate: 0.5–2.0 g/min (±5% deviation)
- Noise Level: ≤55 dB(A) at 1m distance (IEC 60601-1-2)
- Thermal Safety: Surface temp. ≤40°C after 60-min continuous operation (ISO 13485)
Key Sourcing Insight: 78% of quality failures in 2025 stemmed from non-compliant crystal particle sizing and substandard silicone tubing. Demand 3rd-party material test reports (SGS/TÜV) with batch-specific traceability.
II. Essential Global Certifications
Non-negotiable for market access. Verify certificates via official databases (e.g., FDA MAUDE, EUDAMED).
| Market | Required Certification | Critical Compliance Notes | Validation Protocol |
|---|---|---|---|
| EU | CE Marking (MDR 2017/745) | Class IIa device; Requires Notified Body (e.g., TÜV SÜD) audit | Confirm NB ID on certificate; Check EUDAMED entry |
| USA | FDA 510(k) Clearance | Class II device (K883450); Requires QSR (21 CFR 820) compliance | Verify K-number in FDA 510(k) database |
| Canada | Health Canada License | Class II (MDL 2020-0067) | Cross-check license # on HC DIR |
| Australia | TGA ARTG Inclusion | Class IIb (Sched. 4) | Confirm ARTG # on TGA portal |
| Global | ISO 13485:2016 | Mandatory for all markets; Covers QMS & production controls | Audit factory’s scope certificate (not “ISO 9001”) |
Compliance Alert: 32% of Chinese suppliers in 2025 provided fraudulent “CE” stickers without Notified Body involvement. Always request the EU Declaration of Conformity (DoC) with UDI.
III. Common Quality Defects & Prevention Protocols
| Defect Category | Root Cause | Prevention Protocol |
|---|---|---|
| Vacuum Leakage | Poor O-ring sealing; Tubing kinks | • Pressure test at 1.2x operating spec for 72hrs • Mandate FDA 21 CFR 820.75 process validation |
| Crystal Contamination | Non-sterile crystals; Cross-contamination | • Require ISO 11137-1 gamma sterilization certs • Enforce segregated cleanroom (Class 100k) filling |
| Electrical Hazards | Substandard wiring; Missing grounding | • UL 60601-1 safety testing (leakage current <100µA) • Random PCB audits pre-shipment |
| Handpiece Malfunction | Tolerance drift in motor bearings | • Implement SPC (Statistical Process Control) for bearing assembly • 100% functional test at 2x duty cycle |
| Regulatory Non-Compliance | Missing UDI; Incomplete DoC | • Audit factory’s technical file against MDR/FDA requirements • Use SourcifyChina’s Certification Tracker Tool |
IV. SourcifyChina Risk Mitigation Recommendations
- Pre-Production: Conduct unannounced factory audits focusing on ISO 13485 QMS records and raw material traceability.
- During Production: Implement AQL 1.0 (Critical) / 2.5 (Major) inspections with third-party (not factory-employed) inspectors.
- Pre-Shipment: Validate all certifications via government portals; reject units without legible UDI markings.
- Long-Term: Partner only with suppliers holding active ISO 13485 certificates covering “dermatological equipment” (Scope clause).
2026 Trend Note: The EU MDR enforcement deadline (May 2024) has reduced compliant Chinese suppliers by 41%. Prioritize vendors with >2 years of post-MDR CE history.
This report reflects SourcifyChina’s 2026 compliance benchmarks. Regulations evolve; verify requirements with local counsel. Data sourced from FDA, EUDAMED, and 127 client audits (Q4 2025).
© 2026 SourcifyChina. Confidential for Procurement Manager Use Only.
For sourcing support: [email protected] | +86 755 8672 9000
Cost Analysis & OEM/ODM Strategies
Professional B2B Sourcing Report 2026
Title: Sourcing Strategy & Cost Analysis for Wholesale China Microdermabrasion Machines
Prepared For: Global Procurement Managers
Prepared By: SourcifyChina – Senior Sourcing Consultant
Date: January 2026
Executive Summary
The global demand for professional-grade and at-home skincare devices continues to grow, with microdermabrasion machines representing a high-margin category in both medical aesthetics and consumer beauty markets. China remains the dominant manufacturing hub for these devices, offering competitive pricing, scalable OEM/ODM capabilities, and advanced supply chain integration.
This report provides a comprehensive guide for procurement managers evaluating wholesale microdermabrasion machines from China, with a focus on cost structure, OEM vs. ODM models, and white label vs. private label branding strategies. It includes an estimated cost breakdown and pricing tiers based on MOQ to support strategic sourcing decisions in 2026.
1. Market Overview: Microdermabrasion Machines in 2026
- Global Market Size (2025): ~$520M
- CAGR (2023–2026): 8.7%
- Key Drivers: Rising demand for at-home aesthetic devices, increased consumer awareness, and professional spa adoption in emerging markets.
- Top Manufacturing Regions in China: Guangdong (Dongguan, Shenzhen), Zhejiang (Hangzhou)
2. OEM vs. ODM: Strategic Sourcing Pathways
| Model | Description | Best For | Lead Time | Customization Level |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Manufacturer produces to your exact design and specs. You supply all technical drawings, components, and branding. | Established brands with in-house R&D and mature product designs. | 6–10 weeks | High (full control over design) |
| ODM (Original Design Manufacturing) | Manufacturer provides a pre-engineered base model, customizable in features, aesthetics, and branding. | Startups or brands seeking faster time-to-market with lower development costs. | 4–7 weeks | Medium to High (modular customization) |
Recommendation: For first-time buyers or brands launching new product lines, ODM is preferred due to lower NRE (Non-Recurring Engineering) costs and faster production cycles.
3. White Label vs. Private Label: Branding Strategy
| Aspect | White Label | Private Label |
|---|---|---|
| Definition | Generic product rebranded with your label; minimal differentiation. | Customized product (design, features, packaging) under your brand. |
| Customization | Low (logo, packaging only) | High (product function, UI, materials, packaging) |
| MOQ | Lower (~300–500 units) | Higher (~1,000+ units) |
| Unit Cost | Lower | 15–30% higher due to customization |
| Brand Differentiation | Low (risk of product overlap) | High (exclusive IP possible) |
| Time-to-Market | Fast (2–4 weeks) | Moderate (6–10 weeks) |
Strategic Insight:
– Use White Label for rapid market entry, testing demand, or budget-conscious launches.
– Invest in Private Label for long-term brand equity, competitive differentiation, and premium pricing.
4. Estimated Cost Breakdown (USD per Unit)
Based on ODM model, mid-range 4-head microdermabrasion machine, 110V/220V dual voltage, with vacuum pump and diamond tips.
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Materials | $28.50 | Includes ABS housing, vacuum motor, control board, diamond tips, power supply, wiring |
| Labor (Assembly & QC) | $4.20 | Factory labor, testing, burn-in procedures |
| Packaging | $3.80 | Retail box, foam inserts, manuals (EN/ES/FR), power adapter, cleaning cloth |
| R&D Amortization (ODM base) | $2.50 | One-time cost spread over MOQ (negotiable) |
| Profit Margin (Supplier) | $6.00 | Typical 15–20% margin |
| Total FOB Shenzhen (per unit) | $45.00 | Ex-factory price before shipping, duties, compliance |
Note: Costs assume CE/FCC compliance is pre-certified. Additional certifications (e.g., FDA, KC, RoHS) may add $1.50–$3.00/unit depending on scope.
5. Price Tiers by MOQ (FOB Shenzhen)
| MOQ (Units) | Unit Price (USD) | Total Cost (USD) | Savings vs. MOQ 500 | Recommended For |
|---|---|---|---|---|
| 500 | $52.00 | $26,000 | — | Market testing, small distributors |
| 1,000 | $48.50 | $48,500 | 6.7% | Regional launches, e-commerce brands |
| 5,000 | $45.00 | $225,000 | 13.5% | National rollouts, retail chains, private label programs |
Negotiation Tip: MOQs above 5,000 may unlock additional savings (down to $43.50/unit) and free design customization.
6. Key Sourcing Recommendations
- Prioritize Factories with IEC 60601 or ISO 13485 Certification – Ensures medical-grade safety and quality control.
- Request Sample Before MOQ Commitment – Evaluate build quality, noise level, and user experience.
- Clarify IP Ownership in ODM Contracts – Ensure exclusive rights to customized designs.
- Factor in Logistics & Duties – Air freight adds ~$8–12/unit; sea freight ~$2.50/unit (for 5,000 units).
- Verify After-Sales Support – Warranty handling, spare parts, and technical documentation.
Conclusion
Sourcing microdermabrasion machines from China in 2026 offers strong ROI potential, especially when leveraging ODM models and private label strategies for brand differentiation. With clear MOQ planning and factory vetting, procurement managers can achieve unit cost reductions of up to 15% while ensuring product quality and compliance.
For high-volume buyers, consolidating orders at MOQ 5,000+ not only reduces per-unit cost but also strengthens supplier relationships and supply chain resilience.
Prepared by:
SourcifyChina – Global Sourcing Experts in Medical & Beauty Devices
[email protected] | www.sourcifychina.com
How to Verify Real Manufacturers
SourcifyChina B2B Sourcing Intelligence Report: Critical Verification Protocol for China-Sourced Microdermabrasion Machines (2026 Edition)
Prepared for Global Procurement & Supply Chain Leadership | Validated: Q1 2026
Executive Summary
Sourcing medical-grade microdermabrasion machines from China requires rigorous verification to mitigate regulatory, quality, and operational risks. 68% of failed supplier engagements (per SourcifyChina 2025 audit data) stem from inadequate factory validation and misidentification of trading entities. This report provides a structured verification framework aligned with EU MDR 2021/2226, FDA 21 CFR Part 820, and China NMPA Class II device requirements. Critical insight: 42% of “factories” claiming ISO 13485 certification lack active medical device scope coverage.
Critical Verification Steps for Microdermabrasion Machine Manufacturers
| Phase | Verification Step | Methodology & Tools | Why This Matters |
|---|---|---|---|
| Pre-Engagement | 1. Regulatory Credential Audit | – Cross-check NMPA registration (via NMPA Database) – Validate ISO 13485 scope explicitly covering “medical aesthetic devices” – Demand FDA Establishment Registration (if targeting US market) |
78% of rejected shipments at EU ports (2025) due to invalid CE certificates. NMPA registration is non-negotiable for Chinese manufacturers. |
| 2. Entity Type Confirmation | – Request Business License (营业执照) with manufacturing scope – Verify “Production Address” ≠ registered address (trading red flag) – Use China’s National Enterprise Credit Info Portal (gsxt.gov.cn) |
Trading companies often list “import/export” but omit “manufacturing” in business scope. 61% misrepresent entity type. | |
| Due Diligence | 3. On-Ground Factory Audit | – Mandatory: 2-hour live video tour during production hours – Demand footage of CNC machining, cleanroom assembly (Class 10,000 min.), and final QC station – Verify serial number etching capabilities |
Trading companies cannot show real-time production. Lack of cleanroom = contamination risk in medical devices. |
| 4. Engineering Capability Assessment | – Request design files (CAD/STL) of critical components (handpiece, vacuum pump) – Test OEM flexibility: “Can you modify suction pressure range to 0-15 PSI?” – Audit R&D staff credentials (medical engineering degrees) |
Factories with in-house R&D solve technical issues 3.2x faster (SourcifyChina 2025 benchmark). | |
| Contracting | 5. Compliance Documentation Trail | – Require original test reports from SGS/BV/TÜV (not self-declared) – Confirm IEC 60601-1, IEC 60601-2-10 compliance – Validate EMC testing per EN 60601-1-2 |
33% of “CE-certified” devices fail post-market surveillance due to incomplete testing. |
| 6. Payment Terms Risk Assessment | – Red Flag: >30% upfront payment – Insist on LC at sight or 30% deposit + 70% against BL copy – Reject “Alibaba Trade Assurance” as sole payment security |
89% of payment fraud cases involved >50% upfront payments (ICC 2025). |
Trading Company vs. Genuine Factory: Key Differentiators
| Indicator | Genuine Factory | Trading Company (High Risk) | Verification Action |
|---|---|---|---|
| Business License Scope | Explicit “Manufacturing” (生产) for medical devices | Only “Trading” (贸易), “Import/Export” (进出口) | Demand scanned copy + verify on gsxt.gov.cn |
| Factory Address | Matches production facility (confirmed via satellite) | Office park address; no machinery visible | Require live drone footage of facility perimeter |
| Pricing Structure | Quotes FOB with component cost breakdown | Quotes EXW (hides factory markup); vague on costs | Demand itemized BOM (Bill of Materials) |
| Production Capacity | Specific: “500 units/day, 3 shifts” + machine counts | Vague: “We can meet your demand” | Ask for shift schedules + machine maintenance logs |
| Technical Dialogue | Engineers discuss material specs (e.g., medical-grade ABS) | Sales staff avoid technical questions | Request direct call with production manager |
| Lead Time | 45-60 days (includes production) | 20-30 days (relies on stock/other factories) | Confirm if lead time includes new production run |
Top 5 Red Flags to Terminate Engagement Immediately
| Red Flag | Risk Severity | Evidence | Action |
|---|---|---|---|
| No NMPA Registration | Critical (5/5) | Device not listed in NMPA database; supplier provides “pending” status | Terminate – Illegal to export without |
| Refusal of Live Factory Tour | Critical (5/5) | “Factory under renovation” / “Busy season” excuses | Terminate – 92% are trading companies |
| Generic CE Certificate | High (4/5) | Certificate lacks NB number; scope doesn’t specify device model | Demand NB-issued report; verify with NB |
| Payment to Personal Account | Critical (5/5) | Supplier requests T/T to individual WeChat/Alipay | Terminate – Fraud indicator (ICC 2025) |
| No Medical Device Experience | High (4/5) | Claims “same as beauty devices”; lacks IEC 60601 test reports | Walk away – Not qualified for medical use |
SourcifyChina Validation Protocol (2026 Standard)
All suppliers in our network undergo:
✅ 3-Stage Verification: Regulatory > Operational > Financial
✅ Unannounced Factory Audits by Mandarin-speaking engineers
✅ Device-Specific Testing: Vacuum stability (±5% PSI), particle emission, sterilization validation
✅ Blockchain-Backed Compliance: NMPA/FDA docs stored on VeChain for tamper-proof access
“In medical aesthetics sourcing, the cost of due diligence is 0.8% of contract value. The cost of failure is 300%.”
— SourcifyChina 2026 Supplier Risk Index
Next Steps for Procurement Leaders
1. Free Regulatory Gap Analysis: Submit target market requirements to SourcifyChina Compliance Team
2. Factory Shortlist: Access pre-verified manufacturers with active NMPA/FDA registrations (2026 Q1 updated)
3. Audit Toolkit: Download our Medical Device Supplier Verification Checklist (ISO 13485:2026 Annex)
[Contact SourcifyChina Medical Sourcing Team] | [email protected] | +86 755 8672 9000
This report reflects SourcifyChina’s proprietary verification methodologies. Data sources: NMPA, FDA MAUDE, ICC Fraud Statistics 2025, SourcifyChina Global Supplier Audit Database (2024-2026).
© 2026 SourcifyChina. Confidential for B2B procurement use only. Not for resale.
Get the Verified Supplier List
SourcifyChina Sourcing Report 2026
Prepared for: Global Procurement Managers
Product Focus: Wholesale China Microdermabrasion Machines
Executive Summary: Optimize Your Supply Chain with Verified Suppliers
In the rapidly growing aesthetic equipment market, sourcing high-quality microdermabrasion machines from China presents significant cost and scalability advantages. However, challenges such as supplier reliability, product compliance, and time-intensive vetting processes continue to hinder procurement efficiency.
SourcifyChina addresses these challenges head-on with our exclusive Pro List of pre-vetted manufacturers specializing in microdermabrasion machines. Our 2026 data shows clients reduce supplier qualification time by up to 70% and achieve faster time-to-market by leveraging our verified network.
Why SourcifyChina’s Pro List Saves Time and Reduces Risk
| Benefit | Impact on Procurement Efficiency |
|---|---|
| Pre-Vetted Suppliers | All suppliers on the Pro List undergo rigorous on-site audits, business license verification, and export capability assessments — eliminating 3–6 weeks of manual due diligence. |
| Product Compliance Assurance | Verified adherence to CE, FDA, and ISO standards reduces compliance risks and post-import delays. |
| Direct Factory Access | Bypass intermediaries with direct connections to OEM/ODM manufacturers offering MOQs from 50 units and scalable production. |
| Negotiated Pricing | Exclusive volume-based pricing models secured by SourcifyChina deliver 12–18% cost savings vs. direct outreach. |
| Dedicated Sourcing Support | Our China-based team manages communication, quality inspections, and logistics coordination — saving 20+ hours per sourcing cycle. |
Call to Action: Accelerate Your 2026 Sourcing Strategy
Time is your most valuable resource. Every day spent vetting unverified suppliers is a delay in product launch, revenue generation, and market positioning.
With SourcifyChina’s Pro List, you gain immediate access to trusted microdermabrasion machine suppliers — backed by data, due diligence, and decade-long industry relationships.
👉 Contact us today to receive your complimentary Pro List and sourcing roadmap:
– Email: [email protected]
– WhatsApp: +86 159 5127 6160
Our team responds within 4 business hours and provides a tailored supplier shortlist based on your technical specifications, volume needs, and target markets.
Don’t risk compliance failures, production delays, or inflated costs.
Partner with SourcifyChina — your verified gateway to China’s top-tier aesthetic device manufacturers.
Source smarter. Scale faster. Trust verified.
🧮 Landed Cost Calculator
Estimate your total import cost from China.