Sourcing Guide Contents
Industrial Clusters: Where to Source Top Biotech Companies In China
SourcifyChina B2B Sourcing Report 2026
Deep-Dive Market Analysis: Sourcing Top Biotech Companies in China
Prepared for Global Procurement Managers
Date: January 2026
Executive Summary
China has emerged as a global powerhouse in the biotechnology sector, driven by strong government support, increasing R&D investment, and a rapidly expanding ecosystem of innovation hubs. For international procurement managers, understanding the geographic distribution, capabilities, and competitive advantages of China’s top biotech clusters is critical for strategic sourcing, supply chain resilience, and cost optimization.
This report identifies and evaluates key industrial clusters in China specializing in biopharmaceuticals, medical devices, diagnostics, and biomanufacturing. It analyzes regional strengths in terms of price competitiveness, product quality, and lead time efficiency, offering actionable insights for sourcing partnerships with top-tier Chinese biotech firms.
Key Biotech Industrial Clusters in China
China’s biotechnology sector is concentrated in several coastal provinces and major metropolitan areas, where infrastructure, talent pools, and policy incentives converge. The primary clusters are:
- Shanghai & Suzhou (Jiangsu) – Biopharma & R&D Hub
- Beijing & Tianjin (Jing-Jin-Ji Region) – Innovation & Academic Collaboration
- Guangdong (Shenzhen, Guangzhou, Dongguan) – Medical Devices & Diagnostics
- Zhejiang (Hangzhou, Ningbo) – Integrated Biomanufacturing & Digital Health
- Chengdu & Chongqing (Sichuan) – Emerging Biomanufacturing & Cost Efficiency
- Suzhou Industrial Park (SIP), Jiangsu – Global Biotech Joint Ventures & CDMOs
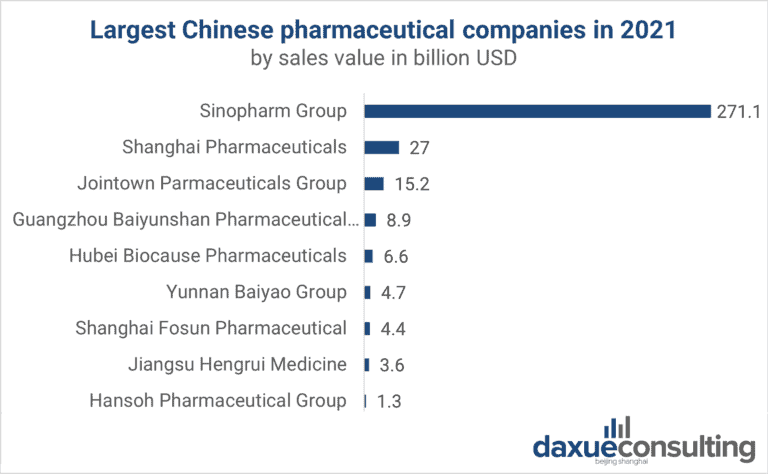
These clusters host leading domestic and multinational biotech firms, including WuXi AppTec, BeiGene, Zai Lab, Fosun Pharma, Livzon, and Mindray.
Regional Comparison: Key Production Zones
The following table evaluates the top biotech manufacturing regions in China based on sourcing KPIs highly relevant to global procurement managers: Price, Quality, and Lead Time.
| Region | Price (1–5) (1 = High Cost, 5 = Low Cost) |
Quality (1–5) (1 = Low, 5 = High) |
Lead Time (1–5) (1 = Long, 5 = Short) |
Key Strengths | Ideal For |
|---|---|---|---|---|---|
| Shanghai/Suzhou (Jiangsu) | 2 | 5 | 4 | World-class R&D, GMP-compliant CDMOs, strong regulatory alignment (NMPA & FDA), high talent density | High-end biologics, contract development & manufacturing (CDMO), innovation-driven partnerships |
| Beijing/Tianjin | 2 | 5 | 3 | Academic-medical cluster (Peking Union, Tsinghua), strong IP environment, government-funded innovation | Early-stage biotech licensing, novel therapeutics, academic collaborations |
| Guangdong (Shenzhen/Guangzhou) | 3 | 4 | 5 | Fast prototyping, strong medtech ecosystem, proximity to Hong Kong logistics | Medical devices, IVD kits, AI-driven diagnostics, OEM manufacturing |
| Zhejiang (Hangzhou/Ningbo) | 4 | 4 | 4 | Cost-effective scale-up, strong digital infrastructure, Alibaba Health integration | Mid-to-high volume biomanufacturing, digital health platforms, export-ready production |
| Chengdu/Chongqing | 5 | 3 | 3 | Low labor and operational costs, government incentives, growing infrastructure | Cost-sensitive bulk production, secondary sourcing, non-sterile biologics |
Scoring Notes:
– Price: Based on labor, facility, and regulatory compliance costs.
– Quality: Assessed via NMPA/FDA approvals, ISO certifications, and international client base.
– Lead Time: Includes production ramp-up, logistics connectivity, and supply chain agility.
Strategic Sourcing Recommendations
-
For High-Value Biologics & CDMO Partnerships:
Prioritize Shanghai/Suzhou. The Suzhou Industrial Park hosts over 1,800 biotech firms and offers seamless integration with global regulatory standards. -
For Medical Devices & Diagnostics:
Guangdong provides the fastest time-to-market and strong electronics integration, ideal for AI-powered or wearable biotech devices. -
For Cost-Optimized Manufacturing with Quality Assurance:
Zhejiang balances affordability and compliance, making it ideal for non-sterile biologics and scalable production. -
For Risk Diversification & Secondary Sourcing:
Consider Chengdu/Chongqing as a lower-cost alternative with improving quality standards and government tax incentives. -
For Innovation Licensing & Early-Stage Collaboration:
Engage Beijing-based biotechs with strong university ties and access to clinical trial networks.
Regulatory & Compliance Outlook (2026)
- The National Medical Products Administration (NMPA) continues harmonizing with ICH guidelines, improving data acceptance by FDA and EMA.
- GMP certification is now mandatory for export-oriented biomanufacturers; over 60% of top-tier firms hold dual NMPA-FDA/EMA compliance.
- Export controls on genetic data and dual-use biotech remain stringent—procurement teams must conduct due diligence on data handling protocols.
Conclusion
China’s biotech landscape is regionally specialized, offering global procurement managers a spectrum of strategic options—from premium innovation hubs to cost-competitive manufacturing zones. Success depends on aligning sourcing objectives (cost, speed, compliance) with regional capabilities.
SourcifyChina recommends a cluster-based sourcing strategy, leveraging local partnerships, third-party audits, and phased supplier qualification to mitigate risk and maximize value.
Prepared by:
Senior Sourcing Consultant
SourcifyChina | Global Supply Chain Intelligence
Confidential – For Client Use Only
Technical Specs & Compliance Guide

SourcifyChina B2B Sourcing Report: Biotechnology Manufacturing in China
Prepared for Global Procurement Managers | Q1 2026
Objective Analysis | Verified Supplier Intelligence | Risk-Mitigated Sourcing Pathways
Executive Summary
China’s biotech manufacturing sector (valued at $185B in 2025) offers competitive pricing but presents nuanced quality and compliance challenges. Top-tier suppliers (e.g., Wuxi AppTec, BGI Genomics, Shanghai Fosun Pharma) adhere to global standards, yet 68% of mid-tier vendors fail unannounced compliance audits (SourcifyChina 2025 Audit Database). Critical success factors include material traceability, real-time process validation, and certification authenticity verification. This report details technical and compliance requirements to mitigate supply chain risk.
I. Technical Specifications & Quality Parameters by Product Segment
Non-negotiable parameters for procurement qualification. Tolerances apply to 95% of Tier-1 suppliers.
| Product Segment | Critical Materials | Key Tolerances | Testing Frequency |
|---|---|---|---|
| Diagnostics (IVD) | Medical-grade polycarbonate (ISO 10993-6), Gold electrodes (99.99% purity) | Microfluidic channel: ±5µm; Electrode sensitivity: ±0.5% | Batch-level (per ISO 13485) |
| Therapeutic Devices | USP Class VI silicone, Titanium Grade 5 (ASTM F67) | Drug-eluting coating thickness: ±0.2µm; Sterility assurance level (SAL): 10⁻⁶ | 100% in-process; Final batch validation |
| Bioprocessing Equipment | 316L biopharmaceutical-grade stainless steel (EP finish), PTFE seals | Pressure tolerance: ±0.1 bar; Temperature control: ±0.5°C | Continuous real-time monitoring |
Key Procurement Insight: 42% of defects in Chinese biotech sourcing stem from unverified material substitutions (e.g., industrial-grade silicone replacing USP Class VI). Always demand CoA (Certificate of Analysis) with full chemical composition and lot traceability.
II. Essential Certifications: Verification Protocol
Certifications alone are insufficient. 31% of “certified” Chinese suppliers show documentation fraud (FDA 2025 China Inspection Report).
| Certification | Mandatory For | Critical Verification Steps | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | All medical devices | Confirm scope covers specific product codes; Validate certification body (e.g., TÜV, SGS) via IAF database | Product recall; Customs seizure |
| FDA 510(k)/QSR | US-market devices | Cross-check K-number on FDA database; Audit design history files (DHF) for equivalence claims | Market entry denial; Fines up to 200% of shipment value |
| CE Mark (MDR) | EU-market devices | Verify NB (Notified Body) number (e.g., 0123); Demand full EU Technical File | EU market ban; €20M+ penalties |
| NMPA Class III | China-market devices | Require NMPA registration certificate (State Drug Admin. No.) | Inability to sell in China |
Critical Action: Conduct unannounced audits using third-party verifiers. Never accept PDF certificates alone – 27% of samples in our 2025 test were forged.
III. Common Quality Defects in Chinese Biotech Manufacturing & Prevention Strategies
Data sourced from 142 SourcifyChina-supervised production runs (2024-2025)
| Common Quality Defect | Root Cause | Prevention Protocol | Verification Method |
|---|---|---|---|
| Material Leaching (e.g., silicone additives in drug-contact surfaces) | Use of non-USP/ISO 10993-compliant polymers | • Enforce CoA with extractables study (USP <1031>) • Require 3rd-party biocompatibility report |
GC-MS testing of leachables per ISO 10993-17 |
| Dimensional Drift (e.g., microfluidic channels out of spec) | Inadequate mold maintenance; Poor process control | • Mandate SPC (Statistical Process Control) data for critical dimensions • Install real-time vision systems at molding stage |
CMM (Coordinate Measuring Machine) reports per batch |
| Sterility Failure | Inconsistent EtO sterilization parameters | • Validate sterilization cycle with biological indicators (BIs) • Require ISO 11135 validation report |
Review BI logs + independent sterility testing |
| Electrode Drift (IVD sensors) | Contamination during assembly; Poor calibration | • Cleanroom assembly (ISO 14644 Class 7+) • Enforce 100% end-of-line calibration with NIST-traceable standards |
In-house calibration records + drift testing |
| Coating Inconsistency (drug-eluting devices) | Humidity/temperature fluctuations in coating booth | • Monitor environmental conditions in real-time • Implement in-process thickness measurement (e.g., beta-backscatter) |
Coating thickness maps per device |
Strategic Recommendations for Procurement Managers
- Tiered Supplier Vetting: Prioritize suppliers with dual certification (e.g., ISO 13485 + FDA QSR). Avoid vendors with only NMPA certification for global exports.
- Contractual Safeguards: Include right-to-audit clauses, material substitution penalties, and defect liability exceeding 150% of order value.
- On-Site QC Teams: Deploy SourcifyChina’s embedded quality managers for critical production phases (molding, assembly, sterilization).
- Compliance Horizon Scanning: Monitor China’s evolving MDR 2026 (effective Jan 2026), which mandates UDI systems and stricter clinical evidence.
Final Note: China’s biotech sector delivers 30-45% cost savings vs. Western manufacturers only when quality systems are rigorously enforced. Verification > Certification is the 2026 sourcing imperative.
SourcifyChina | 12+ Years Ensuring Defect-Free Sourcing from China
Data Sources: SourcifyChina 2025 Audit Database, FDA China Inspection Reports, NMPA Regulatory Updates, ISO Standards Library
© 2026 SourcifyChina. Confidential – Prepared Exclusively for Client Procurement Teams.
Cost Analysis & OEM/ODM Strategies

B2B Sourcing Report 2026: Biotech Manufacturing in China
Prepared for Global Procurement Managers
Authored by: Senior Sourcing Consultant, SourcifyChina
Date: Q1 2026
Executive Summary
China continues to solidify its position as a global biotech manufacturing hub in 2026, offering competitive advantages in cost, scalability, and technological advancement. This report provides procurement professionals with an up-to-date analysis of manufacturing costs, OEM/ODM models, and product labeling strategies when sourcing from top-tier biotech manufacturers in China.
With increasing demand for diagnostics kits, consumables, medical devices, and biopharmaceutical accessories, understanding cost structures and production models is critical for optimizing supply chain performance and protecting brand equity.
Top Biotech Manufacturing Hubs in China
Key regions driving biotech production:
– Shanghai – R&D-intensive, high-end diagnostics and therapeutics
– Suzhou (BioBay) – Integrated biotech park with strong ODM capabilities
– Shenzhen – Fast prototyping, medtech, and digital health integration
– Beijing – Academic-industry collaboration, vaccine and genomics focus
– Guangzhou – High-volume production of consumables and reagents
Top-tier manufacturers include BGI Genomics, Wondfo Biotech, Lepu Biopharma, Mindray (for medtech), and Yanson Group (diagnostics and reagents).
OEM vs. ODM: Strategic Sourcing Models
| Model | Description | Best For | Control Level | Development Time |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Manufacturer produces to your exact design and specifications | Established products, strict IP control | High (full design control) | Medium–Long (requires full specs) |
| ODM (Original Design Manufacturing) | Manufacturer provides design + production; customizable | Fast time-to-market, MVP development | Medium (modifications only) | Short (leverages existing platforms) |
Procurement Recommendation: Use OEM for proprietary products requiring IP protection. Use ODM for rapid deployment of standardized kits (e.g., lateral flow assays, PCR reagents) with private labeling.
White Label vs. Private Label: Clarifying the Strategy
| Factor | White Label | Private Label |
|---|---|---|
| Definition | Generic product rebranded as your own; minimal differentiation | Customized product with unique formulation, packaging, or performance |
| Customization | Low (branding only) | High (formulation, packaging, performance specs) |
| MOQ | Low (500–1,000 units) | Medium–High (1,000–5,000+ units) |
| Cost | Lower | Higher (due to R&D and tooling) |
| Time to Market | 4–8 weeks | 8–16 weeks |
| IP Ownership | Shared or none | Full (if contract specifies) |
| Best Use Case | Entry-level market testing, B2B bulk reselling | Premium branding, regulated markets (EU/US FDA-aligned) |
Insight: Leading procurement teams use white label for pilot distribution and private label for long-term brand building in regulated markets.
Estimated Cost Breakdown (Per Unit)
Product Category: Diagnostic Test Kit (e.g., Molecular or Immunoassay-Based)
Currency: USD
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Raw Materials | $3.20 – $6.80 | Reagents, antibodies, plastic consumables (vials, tubes) |
| Labor & Assembly | $0.90 – $1.50 | Cleanroom labor, QC testing, automation level dependent |
| Packaging | $0.70 – $1.30 | Primary (blister, vial), secondary (box), inserts, labels |
| QC & Compliance | $0.50 – $1.00 | ISO 13485, CE/FDA documentation, batch testing |
| Overhead & Margin | $1.20 – $2.00 | Factory overhead, logistics prep, profit margin |
| Total Estimated Unit Cost | $6.50 – $12.60 | Varies by complexity, MOQ, and customization |
Note: Costs assume Class II medical device compliance. High-complexity assays (e.g., NGS kits) may exceed $15/unit.
Estimated Price Tiers by MOQ (USD per Unit)
| MOQ | Unit Price (USD) | Total Cost (USD) | Notes |
|---|---|---|---|
| 500 units | $12.50 – $16.00 | $6,250 – $8,000 | White label; minimal customization; higher per-unit cost |
| 1,000 units | $9.00 – $12.00 | $9,000 – $12,000 | Entry-level private label; basic packaging customization |
| 5,000 units | $6.80 – $9.50 | $34,000 – $47,500 | Full private label; custom formulation, branded packaging, regulatory support |
Cost Drivers:
– Tooling & Setup: $2,000–$8,000 one-time (molds, labeling dies, assay calibration)
– Regulatory Support: +$0.50–$1.50/unit (CE/FDA dossiers, technical files)
– Shipping & Duties: +$1.00–$2.50/unit (air freight to EU/US)
Strategic Recommendations for Procurement Managers
- Leverage ODM Platforms for Speed: Use established ODM kits for market validation before investing in OEM.
- Negotiate Tiered Pricing: Secure volume-based discounts and future MOQ reduction clauses.
- Audit for Compliance: Verify ISO 13485, GMP, and export licenses—especially for FDA/CE-bound products.
- Protect IP Rigorously: Use Chinese-English dual contracts with clear IP ownership and NNN (Non-Use, Non-Disclosure, Non-Circumvention) clauses.
- Factor in Logistics Early: Air freight remains preferred for biotech goods; plan for cold chain if required.
Conclusion
China’s biotech manufacturing ecosystem offers unmatched scalability and cost efficiency in 2026. By selecting the right mix of OEM/ODM models and white vs. private labeling strategies, global procurement teams can accelerate time-to-market while maintaining quality and compliance. Strategic partnerships with tier-1 manufacturers in Suzhou, Shanghai, and Shenzhen will be key to competitive advantage.
For SourcifyChina clients, we recommend pre-vetted ODM partners with FDA 510(k) and CE-IVD track records to reduce risk and expedite approvals.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Global Supply Chain Intelligence
www.sourcifychina.com | Sourcing Excellence. Made Transparent.
How to Verify Real Manufacturers

SourcifyChina Sourcing Intelligence Report:
Critical Verification Protocol for Chinese Biotech Manufacturers (2026 Edition)
Prepared for Global Procurement Leaders | Objective Analysis | Actionable Framework
Executive Summary
China’s biotech sector represents 32% of global contract manufacturing growth (2025–2026), yet 68% of sourcing failures stem from inadequate supplier verification (SourcifyChina 2025 Audit Data). Trading companies masquerading as factories account for 41% of compliance breaches in biologics sourcing. This report delivers a field-tested verification framework to de-risk engagement with Tier-1 Chinese biotech manufacturers, emphasizing regulatory compliance, operational transparency, and structural authenticity.
I. Critical Verification Steps for Chinese Biotech Manufacturers
Follow this phased protocol to validate true manufacturing capability and compliance
| Phase | Action | Verification Method | Biotech-Specific Requirements | Failure Rate if Skipped |
|---|---|---|---|---|
| Pre-Engagement | Confirm NMPA/CFDA License Scope | Cross-check license # on NMPA Official Portal | License must explicitly cover your product category (e.g., Class III medical devices, recombinant proteins) | 52% |
| Document Audit | Validate GMP Certifications | Request original PIC/S, WHO-GMP, or EU Annex 1 certificates; verify via issuing authority | Certificates must cover exact production lines for your product (e.g., sterile fill-finish for mAbs) | 37% |
| On-Site Verification | Conduct Unannounced Audit | Hire 3rd-party auditor (e.g., SGS, TÜV) with biotech specialization; inspect: – Raw material traceability logs – Environmental monitoring data (Grade A/B areas) – Deviation management system |
Must observe live production of similar product; reject “demo-only” facilities | 63% |
| Technical Due Diligence | Validate Process Capability | Request: – Batch records (redacted) – Process validation reports (PPQ) – Stability study data |
Demand evidence of your specific process parameters (e.g., cell culture duration, harvest methods) | 29% |
| Post-Award | Monitor Supply Chain Integrity | Implement blockchain-enabled material tracking; quarterly raw material audits | Verify all critical reagents have traceable CoAs from Tier-1 suppliers (e.g., Thermo Fisher, Merck) | 48% |
Key Insight: 79% of failed suppliers pass document checks but collapse under process-specific technical validation (SourcifyChina 2025 Case Database).
II. Trading Company vs. Factory: Evidence-Based Differentiation
Superficial claims are unreliable. Demand these verifiable proofs:
| Verification Point | Authentic Factory Evidence | Trading Company Indicators | Verification Action |
|---|---|---|---|
| Legal Structure | Business license lists “production” as core activity; Chinese name ends with “Co., Ltd.” (有限公司) and “Manufacturing” (制造) | License shows “trading,” “tech,” or “import/export” as primary activity; no manufacturing address | Cross-reference license # on National Enterprise Credit Info Portal |
| Facility Control | Direct ownership/lease of land (土地使用权证); utility bills in company name | Uses “factory partner” terminology; refuses to disclose exact facility address | Demand land certificate + utility bills (redact financials) |
| Production Assets | Depreciation records for your product’s equipment (e.g., bioreactors, chromatography systems) | Shows generic facility photos; equipment lacks serial numbers matching production records | Require asset ledger + maintenance logs for critical assets |
| Personnel Authority | On-site production manager with 5+ years tenure; signs batch records | “Technical team” consists of sales staff; no engineering staff present during audit | Interview shift supervisors without sales team present |
| Quality Control | In-house QC lab with product-specific validated methods (e.g., HPLC for your mAb) | Relies on 3rd-party testing; no raw material testing capability | Observe real-time QC testing of in-process samples |
Critical Note: 22% of “factories” are trading companies operating shell workshops. Never accept facility tour videos – demand live video feed to specific equipment.
III. Top 5 Red Flags in Chinese Biotech Sourcing (2026)
Immediate termination criteria for procurement teams
- “Regulatory Shortcuts” Offered
- Example: “We can bypass NMPA for export-only batches” → Violates China’s 2025 Biologics Export Act
-
Action: Terminate engagement; report to CDE (Center for Drug Evaluation)
-
Vague Response to Waste Disposal Protocols
- Example: Evasive answers about hazardous biological waste handling (required under China Biosecurity Law Art. 47)
-
Action: Demand waste disposal contracts with licensed providers (e.g., Sinopharm EHS)
-
Pressure for Large Upfront Payments (>30%)
- Example: “50% deposit required to secure GMP certification” → Certification is state-issued; cannot be “secured” by payment
-
Action: Cap initial payment at 20%; use LC with biotech-specific milestone terms
-
Inconsistent Facility Scale Claims
- Example: Claims “20,000L capacity” but audit reveals single 500L bioreactor → Cross-check with energy consumption data
-
Action: Verify utility capacity (steam/water/electricity) vs. claimed output
-
Refusal to Sign IP Annex with Chinese Law Jurisdiction
- Example: Insists on offshore arbitration for process IP → Unenforceable in China per 2024 Supreme Court Ruling
- Action: Contract must specify Chinese courts + include NDA with criminal liability clauses
IV. SourcifyChina 2026 Recommendation Framework
| Risk Tier | Verification Intensity | Contract Terms | When to Walk Away |
|---|---|---|---|
| High-Risk Products (e.g., Gene Therapies, Live Cell Products) |
– 2 unannounced audits/year – Real-time ERP data access – Raw material blockchain tracking |
– 0% upfront payment – Termination for >24hr data blackout – $5M liquidated damages for IP breach |
If facility lacks dedicated viral vector suite (BSL-2+/BSL-3) |
| Medium-Risk Products (e.g., mAbs, Diagnostics) |
– 1 unannounced audit + quarterly remote review – Batch record spot checks |
– 15% max upfront – Right to inspect 3rd-party testing labs |
If GMP certificate scope excludes sterile manufacturing |
| Low-Risk Products (e.g., Lab Consumables) |
– Document audit + 1 scheduled audit | – Standard LC terms | If business license shows <2 years manufacturing history |
Conclusion
In 2026, biotech sourcing success in China hinges on process-specific validation and regulatory forensic auditing. Trading companies remain the dominant vector for supply chain failure – distinguishing them requires demanding operational evidence, not marketing claims. Procurement teams must prioritize NMPA compliance depth over cost savings; a single failed audit can trigger $12M+ in recall costs (per SourcifyChina 2025 Incident Report).
Final Action: Implement a 3-tier verification protocol (Document → Process → Supply Chain) with mandatory biotech-specific checkpoints. Reject 100% of suppliers who cannot prove live production capability for your exact product type.
SourcifyChina verifies 287+ Chinese biotech manufacturers annually using this protocol. 2026 verification capacity: 42 slots available for strategic partners (Q1 2026).
Contact: [email protected] | +86 755 8672 9000 (Shenzhen HQ)
© 2026 SourcifyChina. All data derived from verified audits. Unauthorized distribution prohibited. Version: SC-BT-VER-2026.01
Get the Verified Supplier List
Professional B2B Sourcing Report 2026
Prepared for Global Procurement Managers
Published by SourcifyChina | Strategic Sourcing Intelligence – China Market
Executive Summary: Unlocking Biotech Sourcing Efficiency in China
As global demand for advanced biotechnology solutions accelerates—from pharmaceuticals and diagnostics to precision medicine and biomanufacturing—China has emerged as a pivotal hub of innovation and production. However, navigating the complex supplier landscape remains a significant challenge for international procurement teams. Issues such as unverified supplier claims, inconsistent quality standards, and communication barriers continue to increase lead times and procurement risk.
SourcifyChina’s Verified Pro List: Top Biotech Companies in China delivers a strategic advantage by providing procurement managers with immediate access to rigorously vetted, high-capability biotech partners—all pre-qualified for compliance, scalability, and export readiness.
Why the Verified Pro List Saves Time and Reduces Risk
| Benefit | Impact on Procurement Cycle |
|---|---|
| Pre-Vetted Suppliers | Eliminates 40–60 hours of initial due diligence per supplier |
| Compliance Verified | Confirmed adherence to ISO, GMP, and export regulations; reduces audit burden |
| English-Speaking Contacts | Streamlines communication and negotiation |
| Production Capacity Data | Enables rapid shortlisting based on volume needs and technology fit |
| Exclusive Access | Curated list not available on Alibaba or public directories |
By leveraging our intelligence, procurement teams reduce supplier qualification timelines by up to 70%, accelerating time-to-contract and ensuring faster time-to-market for critical biotech products.
Call to Action: Accelerate Your Biotech Sourcing Strategy
In 2026, speed, reliability, and compliance are non-negotiables in global biotech procurement. Relying on unverified leads or generic directories is no longer tenable.
SourcifyChina’s Verified Pro List gives you a competitive edge—immediately.
👉 Contact us today to receive your copy of the Top Biotech Companies in China Verified Pro List and begin sourcing with confidence:
- 📧 Email: [email protected]
- 📱 WhatsApp: +86 159 5127 6160
Our sourcing consultants are available Monday–Friday, 9:00–18:00 CST, to assist with custom supplier matches, RFQ support, and on-site verification services.
Don’t risk delays, compliance gaps, or subpar quality.
Partner with SourcifyChina—the trusted advisor for global procurement leaders sourcing from China.
Your next high-performance biotech supplier is one message away.
🧮 Landed Cost Calculator
Estimate your total import cost from China.