The global demand for hemp and cannabis extracts has surged in recent years, driven by expanding legalization, growing consumer interest in wellness products, and advancements in extraction technologies. According to Grand View Research, the global CBD oil market was valued at USD 4.8 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 21.1% from 2023 to 2030. This rapid expansion has intensified the need for efficient, scalable extraction methods—particularly solventless techniques like rosin pressing, which rely heavily on high-performance tincture (rosin) presses. As small-scale producers and commercial manufacturers alike seek reliable equipment to maximize yield and purity, the market for industrial and lab-grade tincture presses has evolved significantly. Today, innovation in pressure control, thermal consistency, and automation is defining the next generation of pressing technology. Based on performance metrics, build quality, and customer feedback, we’ve identified the top seven tincture press manufacturers leading this space.
Top 7 Tincture Press Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
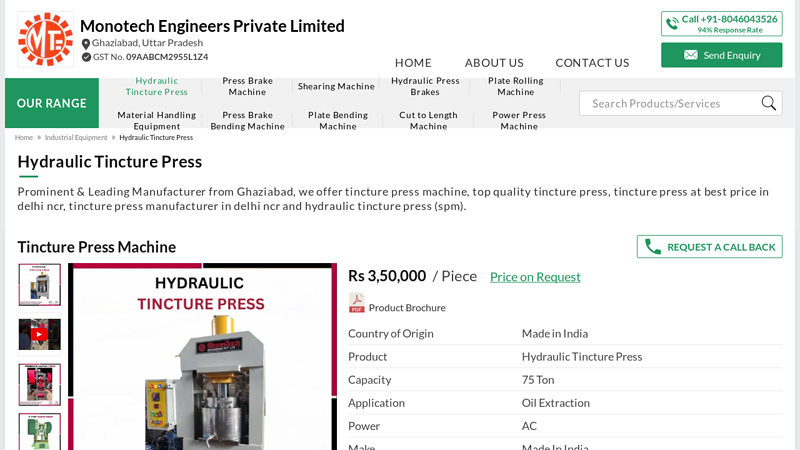
#1 Hydraulic Tincture Press (SPM) Manufacturer from Ghaziabad
Domain Est. 2010
Website: monotechengineers.com
Key Highlights: Our TINCTURE PRESS is purpose-designed for efficient extraction of herbal liquids, ensuring maximum yield and purity. Constructed from food-grade stainless ……
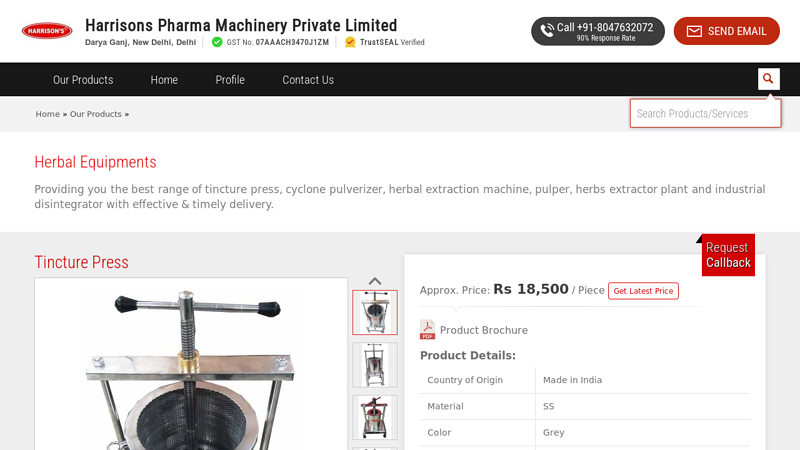
#2 Herbal Equipments
Domain Est. 2012
Website: harrisonsmachinery.com
Key Highlights: Manufacturer of Herbal Equipments – Tincture Press, Cyclone Pulverizer, Herbal Extraction Machine and Pulper offered by Harrisons Pharma Machinery Private ……
#3 Tincture Press Manufacturer,Tincture Press ,Supplier , Exporter, India
Domain Est. 2015
Website: eduteklabscience.com
Key Highlights: EDUTEK INSTRUMENTATION is manufacturer,supplier and exporter of Tincture Press based in Ambala, India.Our Organization offered supreme quality range of ……
#4 Herb Press
Domain Est. 1998
Website: prosysfill.com
Key Highlights: HP1200 Herb Press is for extracting herbal tinctures, oils, extracts and other herbal products for the CBD, Botanical, Nutraceutical & Medical industries….
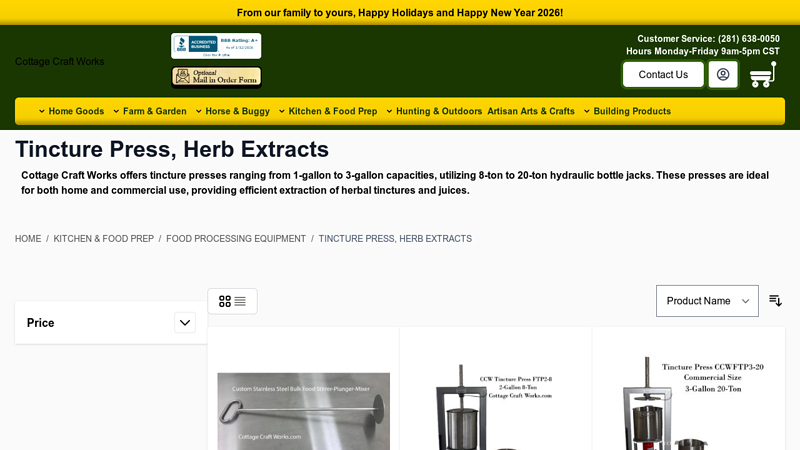
#5 Tincture Press, Herb Extracts
Domain Est. 2007
#6 Wilburs Tincture Press
Domain Est. 2019
Website: wilburstincturepress.com
Key Highlights: It presses and filters your herbal product in one step. It is suitable for the home herbalist, as well as for the manufacturing or school professional who needs ……
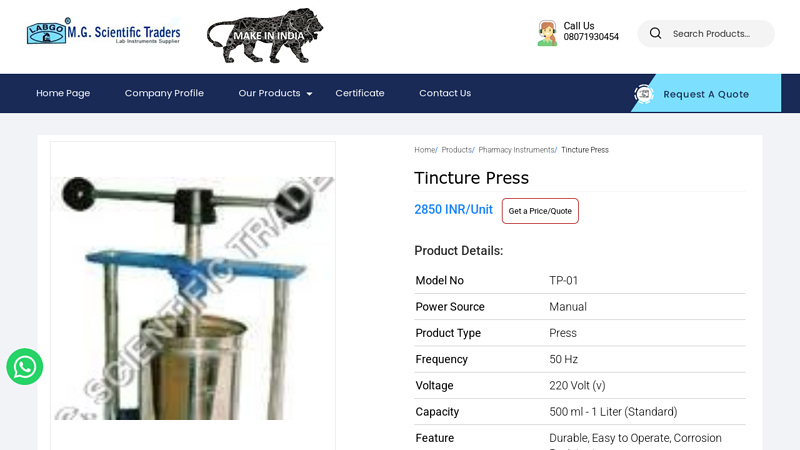
#7 High Quality Manual Tincture Press at Affordable Prices
Domain Est. 2021
Website: scientificinstrumentsworld.com
Key Highlights: Explore our Manual Tincture Press, designed with durable stainless steel construction, precision craftsmanship, and performance optimized for laboratory and ……
Expert Sourcing Insights for Tincture Press

2026 Market Trends for Tincture Press
1. Rising Demand for Precision Extraction Fuels Tincture Press Adoption
By 2026, the herbal and botanical extract market is projected to grow at a CAGR of 8.5%, driven by consumer preference for standardized, high-potency tinctures. Tincture presses will benefit from this trend as manufacturers seek reliable, consistent output. The ability of modern hydraulic and pneumatic presses to extract up to 30% more yield compared to traditional methods positions them as essential tools in commercial and small-batch production, particularly in the CBD, adaptogenic, and functional wellness sectors.
2. Automation and Smart Technology Integration
The integration of IoT-enabled sensors and programmable logic controllers (PLCs) into tincture presses is expected to accelerate by 2026. These advancements allow for real-time pressure monitoring, automated cycle control, and data logging—critical for compliance with GMP (Good Manufacturing Practices) standards. Smart presses will enable producers to maintain batch consistency and reduce human error, appealing to both contract manufacturers and vertically integrated wellness brands.
3. Sustainability and Energy Efficiency as Competitive Advantages
Environmental regulations and consumer demand for eco-conscious production will drive innovation in energy-efficient tincture press designs. By 2026, manufacturers are expected to prioritize low-power hydraulic systems and recyclable material construction. Brands using sustainable extraction methods may leverage this in marketing, with tincture press efficiency contributing to lower carbon footprints across the supply chain.
4. Expansion into Alternative Botanicals and Functional Ingredients
Beyond cannabis and hemp, the surge in interest in medicinal mushrooms (e.g., lion’s mane, reishi), Ayurvedic herbs (ashwagandha, tulsi), and nootropics will expand the tincture press market. Equipment adaptable to diverse plant matrices—offering adjustable pressure settings and compatibility with both fresh and dried botanicals—will be in higher demand. Modular press designs that support multi-stage extraction workflows will gain traction.
5. Regulatory Harmonization and Standardization Pressures
As global regulatory frameworks for herbal supplements and cannabinoid products evolve, standardization in extraction practices will increase. Tincture presses that support traceability, documentation, and reproducible extraction parameters will be favored. Equipment suppliers may partner with testing labs to validate output potency and purity, aligning with FDA and EMA guidelines expected to tighten by 2026.
6. Growth of Home and Micro-Production Markets
The DIY wellness movement and rising interest in personalized herbal medicine will continue to drive demand for compact, user-friendly tincture presses in the consumer and artisanal markets. By 2026, mid-tier desktop models with safety features and simplified operation are expected to capture significant market share, supported by online education platforms and e-commerce sales.
In summary, the 2026 landscape for tincture presses will be defined by technological sophistication, regulatory alignment, and diversification across applications—positioning advanced pressing systems as critical infrastructure in the evolving botanical extract economy.

Common Pitfalls When Sourcing a Tincture Press (Quality, IP)
Sourcing a tincture press—especially for herbal extracts, CBD, or pharmaceutical applications—requires careful attention to both quality and intellectual property (IP) considerations. Overlooking these areas can lead to operational inefficiencies, legal risks, and compromised product integrity.
Quality-Related Pitfalls
Inadequate Material Standards
Many low-cost tincture presses are constructed from subpar materials such as non-food-grade stainless steel or components prone to corrosion. This can result in contamination of sensitive botanical extracts and failure to meet regulatory requirements (e.g., FDA, cGMP).
Poor Pressure and Temperature Control
Consistent extraction relies on precise pressure and temperature management. Sourcing presses without calibrated controls or adequate build quality can lead to inconsistent yields, degraded active compounds, and batch variability.
Lack of Validation and Testing Documentation
Suppliers may not provide performance data, validation reports, or third-party testing results. Without these, it’s difficult to confirm the press’s efficacy, durability, or compatibility with specific plant matrices.
Insufficient After-Sales Support and Spare Parts
Some manufacturers, particularly overseas, offer little technical support or availability of replacement parts. Downtime due to breakdowns can significantly impact production schedules and ROI.
Intellectual Property (IP) Risks
Sourcing from Suppliers with Questionable IP Ownership
Some manufacturers may copy patented press designs or use proprietary technologies without licensing. Purchasing such equipment exposes the buyer to potential infringement claims, especially in regulated markets.
Absence of IP Protection in Contracts
Failure to include clear IP clauses in procurement agreements can mean losing rights to custom modifications or process improvements developed during use. This is critical if the press is adapted for unique formulations or high-throughput workflows.
Reverse Engineering and Trade Secret Exposure
Working with unveted suppliers—especially in regions with weak IP enforcement—increases the risk that your process parameters, material formulations, or equipment tweaks could be reverse-engineered or shared with competitors.
Unclear Warranty and Liability for IP Infringement
Many supplier contracts do not indemnify the buyer against IP lawsuits. If the press incorporates infringing technology, the end user—not the manufacturer—may bear legal and financial responsibility.
Mitigation Strategies
To avoid these pitfalls, conduct thorough due diligence on suppliers, request material certifications and test data, verify IP ownership, and ensure contracts include warranties, indemnification, and clear service-level agreements. Whenever possible, prioritize suppliers with proven track records in regulated industries and robust compliance frameworks.

Logistics & Compliance Guide for Tincture Press
This guide outlines key logistics and compliance considerations for Tincture Press, a company involved in the production and distribution of herbal tinctures. Adhering to these standards ensures product safety, regulatory compliance, and efficient operations.
Regulatory Compliance
Tincture Press must comply with all federal, state, and local regulations governing the manufacture, labeling, and sale of botanical products. Key regulatory frameworks include:
- FDA Regulations: Herbal tinctures are regulated as dietary supplements under the Dietary Supplement Health and Education Act (DSHEA). Compliance includes adherence to Current Good Manufacturing Practices (cGMPs) outlined in 21 CFR Part 111.
- Labeling Requirements: All product labels must include the Supplement Facts panel, ingredient list, net quantity, and a disclaimer statement: “These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
- DSHEA and Adverse Event Reporting: Tincture Press is required to report serious adverse events to the FDA within 15 business days of receipt.
- State Licensing: Ensure proper business and manufacturing licenses are obtained in each state of operation, especially those with specific herbal product regulations (e.g., California Prop 65 compliance).
Ingredient Sourcing & Quality Control
Maintaining product integrity begins with responsible sourcing and rigorous quality assurance.
- Supplier Verification: All botanical suppliers must be vetted for Good Agricultural and Collection Practices (GACP) compliance. Certificates of Analysis (CoA) are required for every incoming ingredient.
- Testing Protocols: Conduct third-party testing for identity, potency, heavy metals, microbial contamination, and residual solvents (especially if ethanol is used in extraction).
- Batch Traceability: Implement a lot-tracking system to trace raw materials through finished goods, enabling rapid recalls if necessary.
Manufacturing & Facility Standards
Facility operations must meet cGMP standards to ensure product consistency and safety.
- Facility Requirements: Maintain clean, organized, and pest-free production areas with appropriate environmental controls (temperature, humidity).
- Equipment Sanitation: Follow documented sanitation procedures and maintain cleaning logs for all processing equipment.
- Personnel Training: Staff involved in manufacturing must receive annual training on hygiene, GMPs, and standard operating procedures (SOPs).
Packaging & Labeling Compliance
Accurate and compliant packaging is essential for consumer safety and legal adherence.
- Child-Resistant Packaging (CRP): Required if products contain alcohol above certain thresholds or are marketed in states with specific CRP laws (e.g., for liquid supplements).
- Expiration Dating: Clearly display “Best By” or expiration dates based on stability testing.
- Allergen & Alcohol Disclosure: If ethanol is used, include “Contains: Alcohol” on the label. Disclose any allergens per FDA guidelines (e.g., soy, gluten if used in carriers).
Distribution & Shipping Logistics
Efficient and compliant distribution ensures products reach customers safely and on time.
- Cold Chain Considerations: While most tinctures are shelf-stable, monitor temperature-sensitive formulations during transit, especially in extreme climates.
- Carrier Compliance: Use carriers that comply with DOT regulations for shipping alcohol-containing products, if applicable.
- Shipping Documentation: Maintain records of shipment dates, batch numbers, and delivery confirmations for traceability.
Recordkeeping & Audits
Robust documentation supports compliance and operational transparency.
- Retention Period: Retain manufacturing records, CoAs, labeling proofs, and adverse event reports for a minimum of three years post-distribution.
- Internal Audits: Conduct quarterly audits of SOPs, facility conditions, and compliance documentation.
- Third-Party Audits: Schedule annual audits by independent GMP-certified auditors to validate compliance.
Environmental & Sustainability Practices
Tincture Press is committed to minimizing environmental impact.
- Sustainable Sourcing: Prioritize organic, ethically wildcrafted, or cultivated botanicals with minimal ecological disruption.
- Waste Management: Properly dispose of solvent waste (e.g., ethanol) per EPA and local hazardous waste regulations.
- Recyclable Packaging: Use glass bottles and recyclable labels and boxes to reduce environmental footprint.
Conclusion
By adhering to this Logistics & Compliance Guide, Tincture Press ensures the production of high-quality, safe, and legally compliant herbal tinctures. Continuous monitoring, staff training, and regulatory vigilance are essential to maintaining trust and operational excellence.
Conclusion:
After evaluating various factors such as cost, capacity, durability, press mechanism, scalability, and supplier reputation, it is evident that selecting the right tincture press is crucial for efficient and high-quality herbal extraction. The ideal sourcing decision should balance performance with long-term value, ensuring compatibility with current production needs while allowing room for growth. Whether opting for a manual, hydraulic, or automated system, choosing a press from a reputable manufacturer with strong technical support and industry experience will enhance extraction efficiency, product consistency, and overall operational success. Ultimately, investing in a reliable tincture press not only improves yield and purity but also supports sustainable, scalable production in the evolving botanical and wellness markets.