The global laboratory equipment market is experiencing steady growth, driven by rising investments in life sciences research, increasing pharmaceutical R&D activities, and expanding biotechnology sectors. According to Mordor Intelligence, the laboratory equipment market was valued at USD 63.8 billion in 2023 and is projected to grow at a CAGR of over 5.8% during the forecast period 2024–2029. As foundational tools in laboratories worldwide, test tube racks play a critical role in sample organization, safety, and workflow efficiency. With increased demand for precision, durability, and ergonomic design, manufacturers are innovating rapidly to meet the evolving needs of academic, clinical, and industrial laboratories. In this competitive landscape, six manufacturers have emerged as leaders, combining quality, scalability, and technological advancement to support modern laboratory operations.
Top 6 Test Tube Rack Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
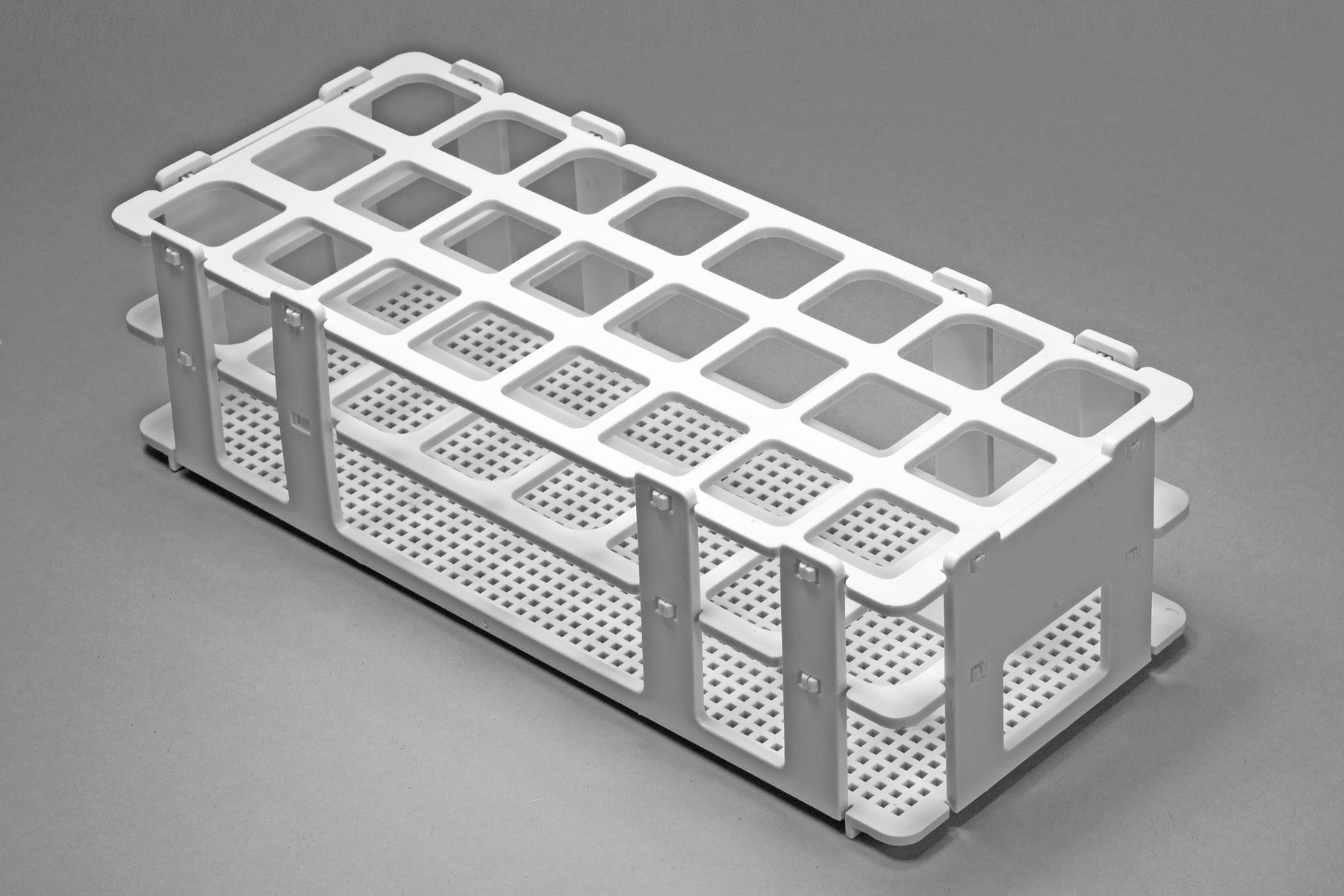
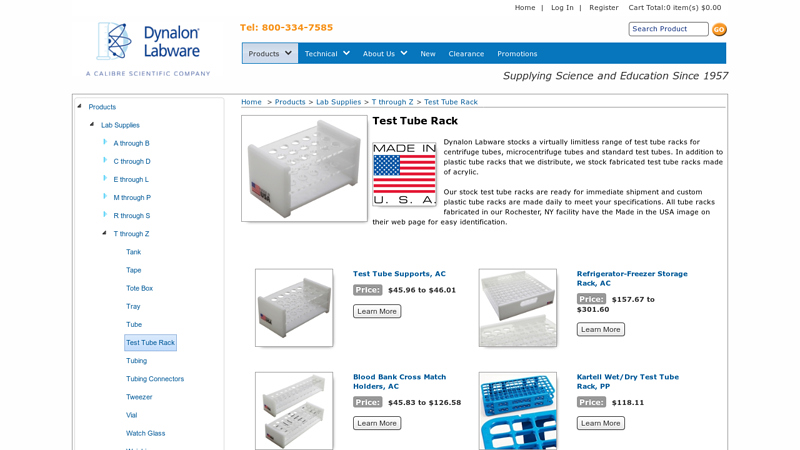
#1 Test Tube Racks
Domain Est. 1995
Website: thomassci.com
Key Highlights: 1–2 day delivery 30-day returnsTest tube racks and microcentrifuge tube racks are available in various sizing to accommodate a range of tube diameters….
#2 Test Tube Racks from Globe Scientific
Domain Est. 1997
Website: globescientific.com
Key Highlights: Globe Scientific offers a complete selection of test tube racks that are offered in plastic, cardboard and stainless steel versions. Racks are available in ……
#3 Tube Racks
Domain Est. 1998
Website: chemglass.com
Key Highlights: Racks with grippers, low profile, 50-place, for up to 17mm tubes. Test tube. In-Stock. Racks with grippers, with tube ejectors, 50-place, for up to 16mm tubes….
#4 Test Tube Racks
Domain Est. 1998
#5 Test Tube Rack
Domain Est. 1999
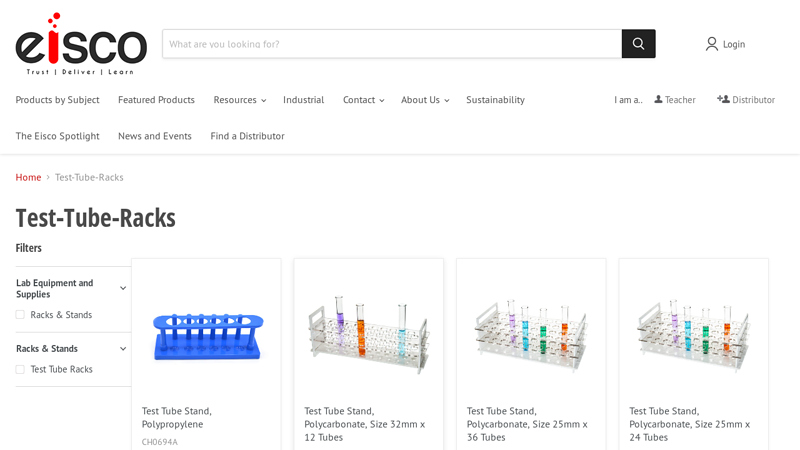
#6 Test
Domain Est. 2000
Website: eiscolabs.com
Key Highlights: Test Tube Rack, Polypropylene, 13mm x 90 Tubes – Eisco Labs Plastic test tube rack Holds 90 test tubes up to 13mm Autoclavable and durable Easy disassembly for ……
Expert Sourcing Insights for Test Tube Rack

H2: Projected 2026 Market Trends for Test Tube Racks
The global test tube rack market is poised for steady growth through 2026, driven by rising demand across research laboratories, clinical diagnostics, pharmaceutical development, and academic institutions. Several key trends are expected to shape the market landscape in the coming years:
-
Expansion of Biotechnology and Pharmaceutical R&D
The continued growth in biotechnological innovation and drug discovery initiatives is fueling demand for high-quality laboratory consumables, including test tube racks. As investment in life sciences R&D increases—particularly in regions like North America, Europe, and emerging Asian markets—laboratories are upgrading their infrastructure, favoring durable, contamination-resistant racks made from materials like polypropylene and stainless steel. -
Shift Toward Ergonomic and Modular Designs
Laboratories are increasingly prioritizing efficiency and safety, leading to a surge in demand for ergonomic, modular, and stackable test tube racks. These designs support high-throughput workflows, optimize bench space, and reduce the risk of sample contamination or spillage. Customizable configurations that accommodate various tube sizes (including microcentrifuge, culture, and cryogenic tubes) are becoming a competitive differentiator among manufacturers. -
Rise in Automation and Compatibility Needs
With the integration of automation in laboratories, there is growing demand for test tube racks compatible with robotic handling systems and automated sample processors. Racks designed to meet ANSI/SLAS footprint standards are seeing increased adoption, ensuring seamless integration with liquid handlers, incubators, and storage systems. This trend is particularly prominent in high-volume clinical testing and pharmaceutical screening environments. -
Sustainability and Reusability
Environmental concerns are pushing laboratories to adopt reusable and autoclavable test tube racks over single-use plastic variants. Manufacturers are responding by offering eco-friendly materials and recyclable products, aligning with institutional sustainability goals. This shift is supported by cost-efficiency motives, as reusable racks offer long-term savings despite higher upfront costs. -
Regional Market Growth and Supply Chain Localization
Asia-Pacific is expected to register the highest compound annual growth rate (CAGR) through 2026, driven by expanding research infrastructure in countries like China, India, and South Korea. Additionally, the post-pandemic emphasis on supply chain resilience is encouraging regional manufacturing of labware, reducing dependence on global imports and enabling faster delivery times. -
Digital Integration and Smart Labware
Though still in early stages, the concept of smart labware—such as racks with embedded RFID tags or QR codes for sample tracking—is gaining interest. This trend supports digital laboratory management systems (LIMS) and enhances traceability, accuracy, and data integrity, particularly in regulated environments.
In summary, the 2026 test tube rack market will be characterized by innovation in design, material science, and integration capabilities. As laboratories evolve toward automation, sustainability, and digitalization, test tube rack manufacturers that offer adaptable, high-performance, and future-ready solutions will be best positioned for growth.

Common Pitfalls When Sourcing Test Tube Racks (Quality and Intellectual Property)
Sourcing test tube racks may seem straightforward, but overlooking key quality and intellectual property (IP) aspects can lead to significant operational, safety, and legal issues. Being aware of these common pitfalls helps ensure reliable laboratory performance and compliance.
Poor Material Quality and Durability
One of the most frequent issues is selecting racks made from substandard materials. Low-quality plastics may warp under high temperatures (e.g., autoclaving), become brittle over time, or leach chemicals into samples. This compromises both sample integrity and laboratory safety. Always verify material specifications—such as resistance to autoclaving, UV exposure, and common solvents—and request certifications like USP Class VI or ISO 10993 for biocompatibility when necessary.
Inconsistent Dimensions and Fit
Inconsistent rack dimensions can result in test tubes not fitting properly, increasing the risk of spills, breakage, or misalignment in automated systems. Poor tolerances may indicate low manufacturing standards. To avoid this, request detailed dimensional drawings and perform incoming inspections or sample testing to confirm compatibility with your specific tube types and equipment.
Lack of Chemical and Heat Resistance Documentation
Many suppliers fail to provide comprehensive data on chemical resistance and thermal stability. Using racks that degrade when exposed to common lab reagents or high temperatures can lead to contamination or equipment damage. Always request and review chemical compatibility charts and thermal performance data before procurement.
Infringement of Intellectual Property (IP)
Some manufacturers produce generic versions of patented or trademarked rack designs, potentially exposing buyers to IP infringement risks. Purchasing counterfeit or copied products—even unknowingly—can result in legal liability, shipment seizures, or reputational damage. Conduct due diligence by verifying original design rights and sourcing from reputable suppliers with transparent IP compliance policies.
Absence of Regulatory Compliance
Depending on the application, test tube racks may need to meet regulatory standards (e.g., FDA, CE, or RoHS compliance). Racks used in clinical or diagnostic settings must often adhere to stricter guidelines. Failing to confirm regulatory status can delay product validation or lead to non-compliance during audits.
Inadequate Packaging and Sterility Assurance
For sterile applications, improper packaging or unreliable sterilization methods (e.g., inconsistent gamma irradiation) can introduce contaminants. Ensure suppliers provide validated sterilization processes and integrity-tested packaging, especially for racks labeled as “sterile.”
By proactively addressing these quality and IP-related pitfalls, organizations can ensure they source test tube racks that are safe, reliable, and legally compliant.
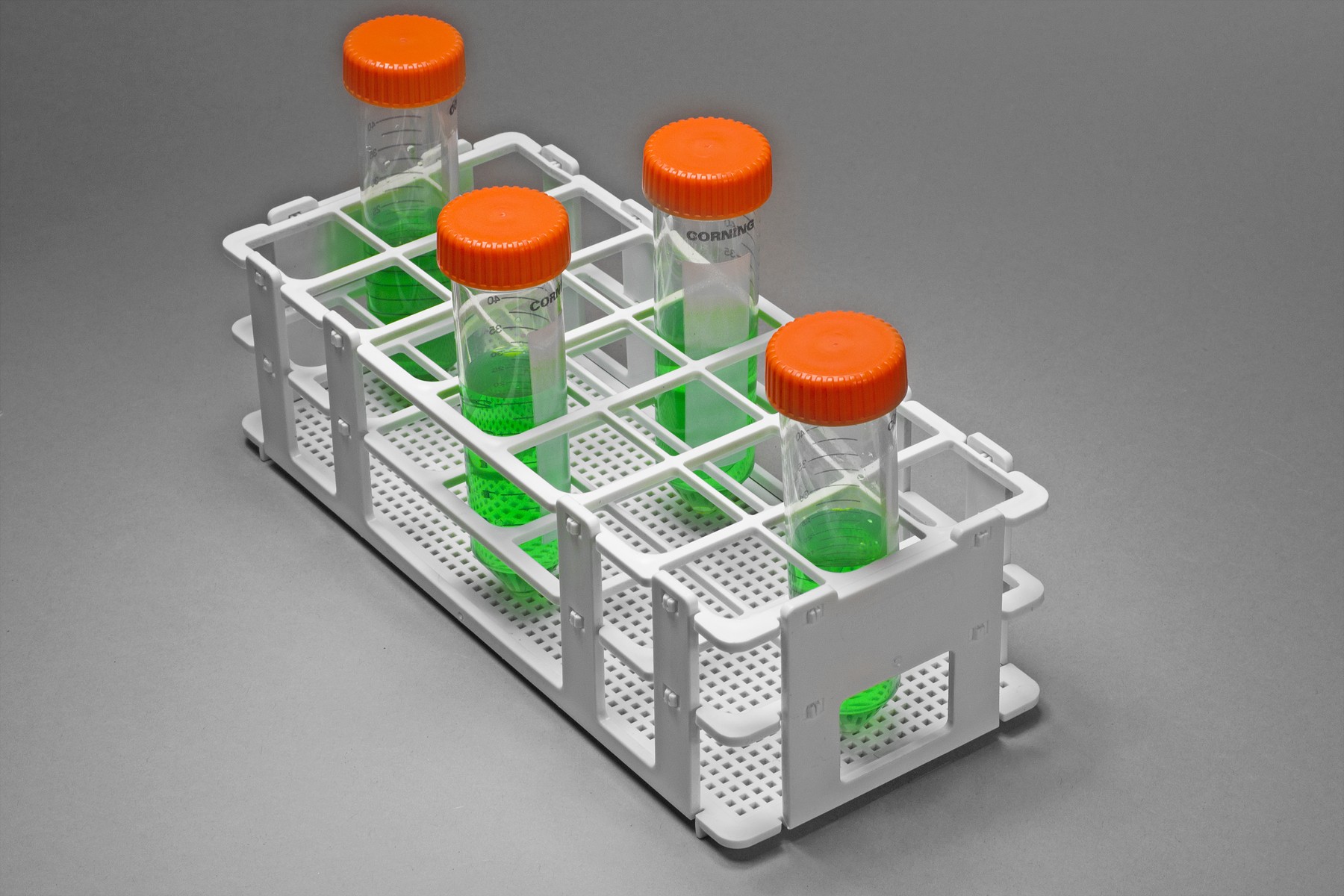
Logistics & Compliance Guide for Test Tube Rack
Product Classification & Documentation
Ensure the test tube rack is accurately classified under the appropriate Harmonized System (HS) code, typically within Chapter 39 (Plastics and Articles Thereof) or Chapter 73 (Articles of Iron or Steel), depending on material composition. Maintain a complete technical dossier including product specifications, material safety data sheets (MSDS/SDS), and certificates of conformity (e.g., ISO 90125 for laboratory equipment, if applicable).
Packaging & Shipping Requirements
Package test tube racks in durable, shock-resistant materials to prevent damage during transit. Use inner dividers or foam inserts if racks are nested or contain accessories. Clearly label packages with handling instructions (e.g., “Fragile,” “This Side Up”) and include a detailed packing list. For international shipments, ensure compliance with ISPM 15 regulations if wooden packaging materials are used.
Regulatory Compliance
Verify compliance with relevant regional and international standards:
– United States: Adhere to FDA guidelines if used in clinical or diagnostic settings; ensure compliance with OSHA handling standards.
– European Union: Meet REACH and RoHS directives regarding restricted substances; affix CE marking if the rack is part of an instrument system requiring conformity.
– Other Regions: Confirm local regulatory requirements for laboratory equipment, especially in countries with strict import controls (e.g., China, Brazil, India).
Import/Export Controls
Determine if the test tube rack is subject to export controls based on intended use. While generally not classified as a controlled item, dual-use concerns may arise if sold to restricted entities or high-risk regions. Conduct end-user screening and maintain records per local export regulations (e.g., U.S. EAR or EU Dual-Use Regulation).
Customs Clearance & Duties
Prepare accurate commercial invoices, certificates of origin, and import permits (if required). Declare the correct HS code, country of manufacture, and value to avoid delays. Be aware of preferential tariff treatments under trade agreements (e.g., USMCA, RCEP) that may reduce duty rates.
Labeling & Traceability
Each test tube rack must bear permanent identification including manufacturer name, model number, material type, and batch/lot number. For sterile or single-use variants, include expiration dates and sterilization method. Labels must be legible and resistant to common lab solvents and autoclaving.
Environmental & Disposal Compliance
Provide guidance for end-of-life disposal in accordance with local waste regulations. Plastics should be marked with resin identification codes. Inform customers of recycling options and avoid labeling products as “biodegradable” unless certified.
Quality Assurance & Recordkeeping
Maintain records of quality inspections, supplier audits, and compliance certifications for a minimum of five years. Implement a traceability system to support recalls or regulatory inquiries. Conduct periodic reviews of logistics partners to ensure ongoing compliance with handling and storage standards.
Conclusion for Sourcing Test Tube Racks:
After evaluating various suppliers, materials, designs, and cost considerations, it is clear that sourcing test tube racks requires a balanced approach that prioritizes quality, durability, functionality, and cost-effectiveness. The ideal test tube rack should meet specific laboratory requirements—such as compatibility with tube sizes, resistance to chemicals and autoclaving, and space efficiency—while also being sustainably and ethically sourced.
Stainless steel and polypropylene emerge as the most reliable materials, each suited to different environmental and usage conditions. Supplier reliability, lead times, and adherence to safety and quality standards are critical factors that influence procurement decisions. Additionally, bulk purchasing and long-term vendor partnerships can lead to significant cost savings without compromising on product integrity.
In conclusion, a well-informed sourcing strategy for test tube racks enhances laboratory efficiency, ensures user safety, and supports operational continuity. By selecting the right combination of product quality and supplier performance, organizations can achieve optimal value and long-term satisfaction in their procurement efforts.