The global stethoscope market is experiencing steady growth, driven by rising demand for diagnostic tools in clinical and educational settings. According to Mordor Intelligence, the global stethoscope market was valued at USD 580.7 million in 2022 and is projected to grow at a CAGR of 6.8% from 2023 to 2028. This expansion reflects increasing enrollments in medical and nursing programs worldwide, fueling demand for high-quality, durable teaching stethoscopes. As institutions prioritize hands-on learning and clinical preparedness, manufacturers are innovating to meet educational needs with cost-effective, acoustically reliable instruments. In this evolving landscape, a select group of manufacturers have emerged as leaders in supplying stethoscopes tailored for teaching environments—balancing performance, durability, and value.
Top 6 Teaching Stethoscope Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Teaching stethoscope
Domain Est. 1997
Website: medicalexpo.com
Key Highlights: Find your teaching stethoscope easily amongst the 15 products from the leading brands (Adam, Rouilly, 3B Scientific, KAWE, …) on MedicalExpo, the medical ……
#2 Student and educator resources
Domain Est. 1997
Website: littmann.com
Key Highlights: Explore more auscultation resources. Join our Stethoscopes for Education Program. Earn Littmann Stethoscopes for your school or student organization….
#3 All Stethoscope Brands
Domain Est. 1997
Website: stethoscope.com
Key Highlights: Free delivery over $125Information about all stethoscope models, colors and parts is online at Stethoscope.com. All models and colors are in stock and available for same day ……
#4 Stethoscopes
Domain Est. 1998
Website: adctoday.com
Key Highlights: … Stethoscopes, General Exam Stethoscopes, Pediatric Stethoscopes, Teaching Stethoscopes, Disposable Stethoscopes, Stethoscope Parts, Stethoscope Accessories ……
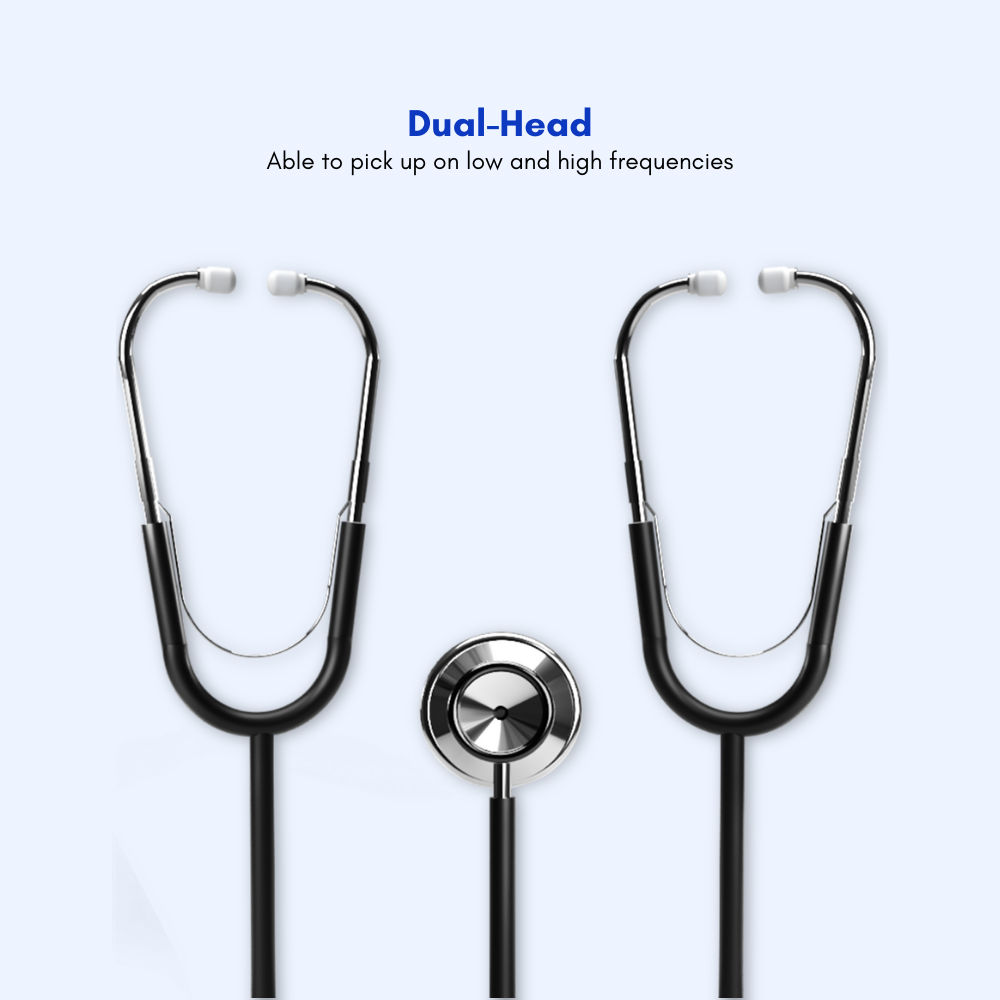
#5 Teaching and Training Stethoscope with Dual Head
Domain Est. 2001
#6 Dual Head Teaching/Training Stethoscope
Domain Est. 2002
Website: aedsuperstore.com
Key Highlights: This dual-head stethoscope is designed specifically for instruction and learning purposes. The teacher and the student can monitor heart sounds simultaneously….
Expert Sourcing Insights for Teaching Stethoscope
2026 Market Trends for Teaching Stethoscopes
The global teaching stethoscope market is poised for significant evolution by 2026, driven by technological advancements, pedagogical shifts, and increasing demand for healthcare education. Key trends shaping the landscape include:
Integration of Digital and Smart Technologies
By 2026, digital teaching stethoscopes with Bluetooth connectivity, real-time sound transmission, and mobile app integration will become standard in medical and nursing schools. These devices enable synchronized auscultation, allowing instructors to broadcast heart and lung sounds to multiple students simultaneously. AI-powered sound analysis and pattern recognition will assist in identifying abnormalities, enhancing diagnostic learning and standardizing training across institutions.
Emphasis on Simulation-Based and Remote Learning
The rise of hybrid and remote education models will accelerate demand for teaching stethoscopes compatible with simulation labs and virtual classrooms. Devices that interface with manikins and electronic learning platforms will dominate, offering real-time feedback and performance tracking. This trend supports scalable, consistent training, especially in underserved or geographically dispersed regions.
Focus on Ergonomics and Accessibility
Manufacturers will prioritize ergonomic design, lightweight materials, and customizable features to accommodate diverse learners, including those with hearing impairments. Stethoscopes with amplified sound, noise-cancellation, and visual soundwave displays will gain traction to improve inclusivity and learning outcomes.
Sustainability and Cost-Effectiveness
Educational institutions will increasingly favor durable, repairable, and eco-friendly teaching stethoscopes. Modular designs that allow part replacement (e.g., ear tips, diaphragms) will reduce waste and long-term costs, aligning with institutional sustainability goals.
Expansion in Emerging Markets
Growing investment in healthcare education in Asia-Pacific, Latin America, and Africa will drive market growth. Affordable, high-quality teaching stethoscopes tailored to local needs will open new opportunities for manufacturers seeking to expand their global footprint.
In summary, by 2026, the teaching stethoscope market will be defined by smart, connected, and inclusive solutions that align with modern medical education paradigms, enhancing both the quality and accessibility of clinical training worldwide.
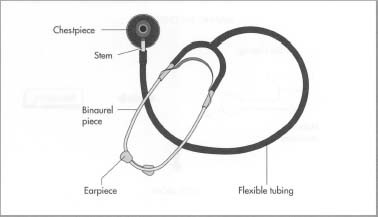
Common Pitfalls When Sourcing Teaching Stethoscopes (Quality and Intellectual Property)
Sourcing teaching stethoscopes—devices used to allow multiple listeners to hear auscultation sounds simultaneously—can present unique challenges, especially concerning product quality and intellectual property (IP) protection. Avoiding these pitfalls is crucial for maintaining clinical training standards and safeguarding your organization legally and financially.
Poor Acoustic Performance and Build Quality
One of the most prevalent issues is receiving teaching stethoscopes with substandard acoustic fidelity. Unlike regular stethoscopes, teaching models must transmit clear, undistorted sounds to multiple users. Low-quality units often suffer from sound dampening, echo, or frequency loss, which can compromise student learning. Additionally, cheap materials and poor construction can lead to cracked tubing, loose headset connections, or earpiece discomfort—resulting in frequent replacements and higher long-term costs.
Inadequate Noise Isolation
Many lower-tier teaching stethoscopes fail to provide proper noise isolation. Without effective sound channeling, ambient noise can overpower the patient’s physiological sounds, making it difficult for students to discern heart murmurs, breath sounds, or other critical auditory cues. This flaw undermines the educational purpose and leads to inaccurate clinical assessments during training.
Misrepresentation of Brand and Design (IP Infringement)
A significant risk in sourcing, especially from non-reputable suppliers or generic manufacturers, is the unintentional procurement of counterfeit or IP-infringing products. Some suppliers may copy the design, branding, or patented features (e.g., dual-head configurations, specialized tubing layouts) of well-known brands like 3M Littmann without proper licensing. Using such products exposes institutions to legal liability, reputational damage, and potential cease-and-desist orders.
Lack of IP Clearance in Custom Designs
Institutions or suppliers developing custom teaching stethoscopes must ensure that their designs do not infringe on existing patents or trademarks. Overlooking patent searches or failing to secure proper IP rights can result in costly litigation. For example, unique Y-split configurations or amplification mechanisms may be protected by utility patents, and unauthorized use—even in educational settings—can constitute infringement.
Inconsistent Compliance with Medical Device Standards
Teaching stethoscopes, while primarily educational, may still fall under medical device regulations depending on jurisdiction. Sourcing products that do not meet ISO 11952 (acoustics of stethoscopes) or regional safety standards (e.g., FDA, CE marking) can pose compliance risks. Poorly sourced units might lack biocompatible materials or sterilization compatibility, creating hygiene and safety concerns in clinical training environments.
Unreliable Supplier Documentation and Traceability
When sourcing internationally, suppliers may not provide adequate documentation regarding materials, manufacturing processes, or IP ownership. This lack of transparency makes it difficult to verify authenticity, ensure quality control, or defend against IP claims. Always request certificates of conformity, patent licenses (if applicable), and detailed product specifications before finalizing procurement.
Conclusion
To mitigate these risks, institutions should source teaching stethoscopes from authorized distributors or reputable manufacturers with proven quality control and transparent IP practices. Conducting due diligence—including acoustic testing, IP audits, and supplier verification—ensures that educational tools are both effective and legally compliant.

Logistics & Compliance Guide for Teaching Stethoscope
Product Classification and Regulatory Requirements
Teaching stethoscopes are typically classified as Class I medical devices under regulatory frameworks such as the U.S. Food and Drug Administration (FDA) and the European Union’s Medical Device Regulation (MDR). As non-invasive tools used for educational demonstration rather than clinical diagnosis, they generally require minimal regulatory oversight. However, manufacturers and distributors must still ensure compliance with general controls, including proper labeling, registration of the device, and adherence to quality management systems (e.g., ISO 13485). Always verify local regulations in target markets to confirm classification and required documentation.
Labeling and Packaging Standards
All teaching stethoscopes must be clearly labeled with essential information, including the product name, intended use (e.g., “For educational and training purposes only”), manufacturer details, model number, and compliance markings (e.g., CE mark for EU, FDA registration number for U.S. market). Packaging should be durable and include instructions for use, cleaning guidelines, and any relevant safety warnings. If the product contains latex (e.g., in ear tips), a latex warning must be prominently displayed. Multilingual labeling may be required for international distribution.
Import and Export Documentation
When shipping teaching stethoscopes across borders, ensure all required export documentation is prepared, including commercial invoices, packing lists, and certificates of origin. For shipments to regulated markets, provide proof of device registration or declaration of conformity (e.g., EU Declaration of Conformity). Some countries may require import permits or additional certifications—verify destination-specific requirements with customs authorities or a licensed customs broker.
Shipping and Handling Procedures
Use secure, protective packaging to prevent damage during transit. Include cushioning materials and consider environmental factors such as temperature and humidity, especially for long-distance or overseas shipping. Label packages as “Fragile” if necessary. Choose reliable logistics partners with experience in medical or educational equipment distribution to ensure timely and compliant delivery. Maintain tracking and insurance for all shipments.
Quality Assurance and Post-Market Surveillance
Implement a quality control process to inspect stethoscopes before dispatch, checking for defects in tubing, earpieces, and chest pieces. Collect customer feedback and monitor for any product complaints or safety issues. Although Class I devices have lower reporting requirements, maintain records of incidents and conduct periodic internal audits to ensure ongoing compliance with applicable standards.
Environmental and Disposal Compliance
Teaching stethoscopes often contain plastics and metals. Provide end-of-life guidance to users, recommending proper recycling where feasible. Comply with environmental regulations such as the EU’s Restriction of Hazardous Substances (RoHS) directive, ensuring no restricted materials (e.g., lead, cadmium) are used above allowable limits. For institutions, support take-back programs or responsible disposal partnerships where available.
Training and User Compliance
Distribute user manuals that emphasize the educational purpose of the device and discourage clinical use unless specifically approved. Include guidance on hygiene practices, such as cleaning earpieces and tubing with mild soap and water or approved disinfectants. Offer digital resources or training sessions to educators on proper handling and maintenance to extend product life and ensure safe use in classroom environments.
Conclusion for Sourcing a Teaching Stethoscope:
Sourcing a high-quality teaching stethoscope is a critical step in ensuring accurate auscultation training for medical and healthcare students. After evaluating various models based on acoustic performance, durability, anatomical fit, and educational features, it is evident that selecting a stethoscope specifically designed for instructional use enhances both teaching effectiveness and student learning outcomes. Key considerations such as dual-head design (allowing instructor and student to listen simultaneously), superior sound transmission, and ergonomic construction contribute significantly to clinical skill development. Brands like 3M Littmann offer reliable, clinically proven options that are widely recommended in academic settings. Ultimately, investing in a purpose-built teaching stethoscope supports hands-on learning, fosters confidence in diagnostic abilities, and promotes excellence in patient assessment from the early stages of training.