The global potassium periodate market is experiencing steady growth, driven by rising demand in pharmaceuticals, chemical synthesis, and analytical applications. According to Grand View Research, the global iodine and iodide market—of which potassium periodate is a key derivative—is projected to expand at a CAGR of approximately 4.5% from 2023 to 2030, fueled by increasing use in radiopacity agents, disinfectants, and specialty chemicals. Mordor Intelligence further highlights growing applications in oxidative cleavage reactions and diagnostic reagents as key growth catalysts, particularly in mature and emerging pharmaceutical markets. As demand for high-purity potassium periodate rises across R&D and industrial sectors, a select group of manufacturers have emerged as leaders in quality, scale, and innovation—shaping the competitive landscape of this niche but critical segment of the inorganic chemicals industry.
Top 6 Potassium Periodate Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Potassium Periodate
Domain Est. 2005
Website: williamblythe.com
Key Highlights: We are a leading manufacturer of high purity potassium periodate. Our potassium periodate is used in chemical manufacturing as an oxidiser or in the ……
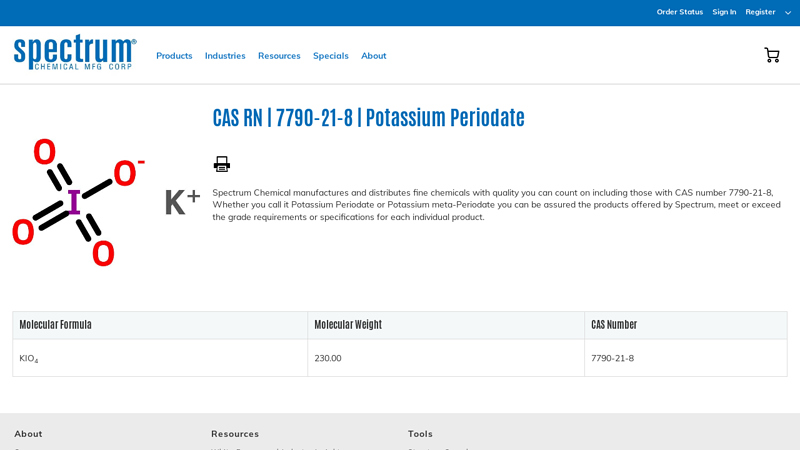
#2 CAS Number 7790-21-8
Domain Est. 1995
Website: spectrumchemical.com
Key Highlights: 15-day returnsPurchase and Find information on CAS 7790-21-8, Potassium Periodate which meets or exceeds the grade requirements or specifications for each fine chemical….
#3 Potassium periodate
Domain Est. 1996
#4 Potassium periodate
Domain Est. 1998
#5 Potassium Periodate
Domain Est. 1998
Website: americanelements.com
Key Highlights: Potassium Periodate is a transparent colorless crystal that is a strong oxidizer. It is generally immediately available in most volumes….
#6 Potassium Periodate, Technical
Domain Est. 2012
Expert Sourcing Insights for Potassium Periodate

H2: Market Trends for Potassium Periodate in 2026
As of 2026, the global market for potassium periodate (KIO₄) is experiencing steady but niche growth, driven primarily by its specialized applications in analytical chemistry, advanced materials, and select industrial processes. The following analysis outlines key market trends shaping the demand, supply, and technological developments related to potassium periodate in 2026.
-
Stable Demand from Analytical and Research Sectors
Potassium periodate remains a critical reagent in analytical laboratories, particularly for the oxidation of glycols and vicinal diols in carbohydrate analysis, and in the spectrophotometric determination of elements such as manganese. In 2026, academic and industrial research institutions in North America, Europe, and parts of East Asia continue to represent the largest consumers. The expansion of life sciences and pharmaceutical R&D has sustained consistent demand, although at relatively low volume due to the compound’s specificity. -
Growth in Specialty Chemical Applications
Emerging applications in polymer chemistry and surface modification technologies are contributing to modest market expansion. Potassium periodate is increasingly used in the synthesis of functionalized polymers and as an oxidizing agent in the development of biosensors and medical diagnostics. These high-value applications are driving interest from specialty chemical manufacturers, particularly in regions with strong biotech sectors such as the U.S., Germany, and South Korea. -
Supply Chain Resilience and Regional Production Shifts
Historically reliant on a limited number of chemical suppliers—mainly in China and Europe—the potassium periodate market has seen a shift toward regional diversification in 2026. Geopolitical tensions and post-pandemic supply chain reforms have prompted companies to secure alternative sourcing strategies. Some U.S. and Indian chemical producers have ramped up domestic production capabilities to reduce dependency on single-source suppliers, enhancing supply chain resilience. -
Price Stability Amid Rising Raw Material Costs
While the prices of raw materials such as iodine and potassium hydroxide have experienced moderate inflation due to energy and mining constraints, potassium periodate prices have remained relatively stable in 2026. This stability is attributed to efficient production processes and the compound’s low consumption volume. However, smaller laboratories and emerging market buyers report tighter margins, prompting interest in cost-effective alternatives or recycling protocols. -
Environmental and Regulatory Considerations
Environmental regulations, particularly in the EU and North America, are influencing handling, disposal, and transportation protocols for oxidizing agents like potassium periodate. The compound is classified as an oxidizer under GHS regulations, and companies are investing in safer formulations and green chemistry alternatives where feasible. Despite this, no major substitutes have emerged due to its unique oxidizing properties, preserving its market position. -
Technological Advancements and Purity Demands
There is a growing demand for ultra-high-purity potassium periodate (99.9%+), especially in semiconductor and nanotechnology applications where trace impurities can affect performance. Leading suppliers have responded by enhancing purification processes, including recrystallization and sublimation techniques, to meet advanced industry standards. -
Limited Competition and Niche Market Dynamics
The potassium periodate market remains fragmented and highly specialized, with few large-scale producers. This lack of competition allows established manufacturers to maintain pricing power, but also limits aggressive innovation. Market entry remains challenging due to technical complexity and regulatory compliance requirements.
Conclusion:
In 2026, the potassium periodate market reflects a stable, niche chemical segment characterized by consistent demand from research and high-tech industries, moderate supply chain adaptations, and ongoing emphasis on purity and safety. While not a high-growth commodity, its irreplaceable role in specific chemical processes ensures continued relevance. Future growth will likely depend on innovation in downstream applications, particularly in biotechnology and advanced materials engineering.

H2: Common Pitfalls When Sourcing Potassium Periodate (Quality and Intellectual Property Considerations)
Sourcing potassium periodate (KIO₄) for industrial, analytical, or research applications requires careful attention to both quality specifications and intellectual property (IP) concerns. Below are common pitfalls to avoid in each category:
1. Quality-Related Pitfalls
- Inadequate Purity Grades: Potassium periodate is available in various purity grades (e.g., laboratory, reagent, technical, or ultra-pure). Using a lower-grade material in sensitive applications (e.g., pharmaceutical synthesis or high-precision analysis) can lead to inconsistent results, contamination, or failed reactions.
Best Practice: Verify the required assay (typically ≥98–99.5%) and impurity profile (e.g., heavy metals, chloride, iodide content) for your application. Request a Certificate of Analysis (CoA) from the supplier.
- Moisture Sensitivity and Stability: Potassium periodate is hygroscopic and may degrade upon exposure to moisture or light, leading to reduced oxidizing capacity.
Best Practice: Ensure packaging is airtight and moisture-resistant (e.g., sealed with desiccants). Store in a cool, dry, dark environment. Confirm shelf life and stability data from the supplier.
- Incorrect Crystalline Form or Hydration State: Some suppliers may offer hydrated forms or mixtures. The anhydrous form is typically required for precise stoichiometry in chemical reactions.
Best Practice: Confirm the chemical form (anhydrous KIO₄) and check for hydration via specification sheets or analytical testing upon receipt.
- Unverified Supplier Credibility: Sourcing from unqualified or unknown suppliers, especially in online marketplaces, increases the risk of receiving mislabeled, adulterated, or substandard material.
Best Practice: Use audited suppliers with proven track records in fine chemicals. Evaluate third-party certifications (e.g., ISO 9001, GMP if applicable).
2. Intellectual Property (IP)-Related Pitfalls
- Patented Synthesis or Application Methods: Potassium periodate may be used in processes protected by patents (e.g., in organic synthesis, pharmaceutical intermediates, or diagnostic assays). Unlicensed use of the compound in a patented process could lead to IP infringement.
Best Practice: Conduct a freedom-to-operate (FTO) analysis before commercializing a process involving potassium periodate. Consult legal counsel to assess relevant patents in your jurisdiction.
- Proprietary Formulations or Graded Products: Some suppliers may market specialty formulations (e.g., stabilized KIO₄ for specific assays) under protected IP. Replicating or reverse-engineering such products without authorization may violate trade secret or patent laws.
Best Practice: Avoid copying branded specialty products. If using a proprietary formulation, ensure proper licensing or opt for generic equivalents where permitted.
- Misrepresentation of “Generic” Availability: Assuming that because potassium periodate is a well-known chemical, all uses are unrestricted, can be misleading. While the compound itself is not typically patentable, its novel uses, formulations, or synthesis routes may be.
Best Practice: Distinguish between the chemical as a commodity and its application-specific uses. Monitor patent databases (e.g., USPTO, Espacenet) for relevant claims.
Conclusion
To mitigate risks when sourcing potassium periodate, prioritize suppliers with rigorous quality control and transparent documentation. Simultaneously, conduct due diligence on IP landscapes related to your intended application. Combining robust supply chain practices with IP awareness ensures both regulatory compliance and operational reliability.

Logistics & Compliance Guide for Potassium Periodate (H2)
Prepared in accordance with GHS and international chemical safety standards
1. Chemical Identification
- Chemical Name: Potassium Periodate
- Synonyms: KIO₄, Potassium metaperiodate
- CAS Number: 7790-21-8
- Molecular Formula: KIO₄
- Molecular Weight: 230.00 g/mol
- UN Number: UN 3085
- Transport Hazard Class: 6.1 (Toxic Substances)
- Packing Group: III (Low to moderate hazard)
- GHS Classification (H2):
- H302: Harmful if swallowed.
- H315: Causes skin irritation.
- H319: Causes serious eye irritation.
- H335: May cause respiratory irritation.
- H412: Harmful to aquatic life with long-lasting effects.
2. Hazard Identification (H2 Phrases)
The following H2 hazard statements apply:
- H302: Harmful if swallowed.
- H315: Causes skin irritation.
- H319: Causes serious eye irritation.
- H335: May cause respiratory irritation.
- H412: Harmful to aquatic life with long-lasting effects.
Precautionary Statements (P-Codes):
– P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
– P270: Do not eat, drink or smoke when using this product.
– P273: Avoid release to the environment.
– P280: Wear protective gloves/protective clothing/eye protection/face protection.
– P302+P352: IF ON SKIN: Wash with plenty of soap and water.
– P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
– P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
– P312: Call a POISON CENTER or doctor/physician if you feel unwell.
– P330: Rinse mouth.
– P391: Collect spillage.
– P501: Dispose of contents/container in accordance with local regulations.
3. Composition and Information on Ingredients
- Potassium Periodate: ≥98% (pure)
- Impurities: May contain trace iodates or chlorides (as per manufacturing process).
4. First Aid Measures
- Inhalation: Move to fresh air. If breathing is difficult, administer oxygen. Seek medical attention if symptoms persist.
- Skin Contact: Remove contaminated clothing. Wash skin thoroughly with soap and water. Seek medical advice if irritation occurs.
- Eye Contact: Immediately flush eyes with copious amounts of water for at least 15 minutes. Consult an eye specialist.
- Ingestion: Rinse mouth. Do NOT induce vomiting. Seek immediate medical attention.
5. Fire-Fighting Measures
- Extinguishing Media: Use water spray, dry chemical, foam, or CO₂.
- Special Hazards: May release toxic fumes (iodine oxides, potassium oxide) when heated.
- Protective Equipment: Wear self-contained breathing apparatus (SCBA) and full protective gear.
- Unusual Fire & Explosion Hazards: Not flammable, but may enhance combustion of other materials (oxidizing agent).
6. Accidental Release Measures
- Personal Precautions: Wear PPE (gloves, goggles, respirator if dust is airborne).
- Environmental Precautions: Prevent entry into drains, waterways, or soil.
- Containment & Cleanup: Use dry, non-combustible material (e.g., sand, vermiculite) to absorb. Collect in a sealed container. Avoid generating dust.
7. Handling and Storage
- Handling: Use only in well-ventilated areas. Avoid dust formation. Do not eat, drink, or smoke during handling.
- Storage:
- Store in a cool, dry, well-ventilated area.
- Keep container tightly closed.
- Store away from reducing agents, combustible materials, and foodstuffs.
- Segregate from acids and organic materials.
8. Exposure Controls and Personal Protection
- Engineering Controls: Local exhaust ventilation if dust is generated.
- Personal Protective Equipment (PPE):
- Eye Protection: Safety goggles or face shield.
- Skin Protection: Wear nitrile or neoprene gloves; lab coat or protective clothing.
- Respiratory Protection: Use NIOSH-approved respirator if airborne concentrations exceed exposure limits.
- Exposure Limits:
- No OSHA PEL or ACGIH TLV established specifically for potassium periodate.
- Follow prudent industrial hygiene practices; treat as toxic.
9. Physical and Chemical Properties
- Appearance: White crystalline powder
- Odor: Odorless
- Melting Point: Decomposes at ~582°C
- Solubility in Water: ~8.3 g/100 mL at 20°C
- Density: ~3.6 g/cm³
- pH (1% solution): ~6–8 (neutral to slightly acidic)
10. Stability and Reactivity
- Stable under normal conditions.
- Conditions to Avoid: High temperatures, moisture (for long-term storage).
- Incompatible Materials: Strong reducing agents, combustible materials, organic compounds, acids (may release toxic gases).
- Hazardous Decomposition Products: Iodine oxides, potassium oxide, oxygen (when heated).
11. Toxicological Information
- Acute Toxicity (Oral, rat): LD50 ~1000–2000 mg/kg (moderately harmful).
- Skin Irritation: Mild to moderate.
- Eye Irritation: Severe (based on similar compounds).
- Respiratory Sensitization: Not reported.
- Chronic Effects: Prolonged exposure may affect thyroid function due to iodine content.
12. Ecological Information
- H412: Harmful to aquatic life with long-lasting effects.
- Bioaccumulation: Low potential, but iodine may affect aquatic ecosystems.
- Persistence: Stable in water; may slowly hydrolyze.
13. Disposal Considerations
- Waste Code: Consult local regulations (e.g., EPA, EU Waste Framework Directive).
- Disposal Method: Neutralize if possible, then dispose of as hazardous waste through licensed contractor.
- Do NOT dispose in sewer or environment.
14. Transport Information (UN / IATA / IMDG)
- UN Number: UN 3085
- Proper Shipping Name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Potassium Periodate)
- Hazard Class: 6.1 (Toxic)
- Packing Group: III
- Labels Required: Toxic (6.1), Environmentally Hazardous (fish and tree symbol)
- Special Provisions: Package must be leak-proof and dust-tight.
- IATA/ICAO: Complies with PI 602 for cargo aircraft only (if quantity exceeds limits).
- IMDG: Marine pollutant – yes.
15. Regulatory Information
- TSCA (USA): Listed
- DSL (Canada): Listed
- REACH (EU): Registered
- GHS Implementation: Compliant in EU (CLP), USA (HazCom 2012), and other GHS-adapted regions.
- EPA: Regulated under TSCA and CERCLA (reportable quantity may apply).
16. Other Information
- GHS Pictograms:
(Irritant)
(Acute Toxicity)
-
(Environmental Hazard)
-
Prepared By: [Your Company EHS Department]
- Revision Date: [Insert Date]
- Reference: SDS Format in accordance with ISO 11014 and GHS Rev. 9.
Note: Always consult the full Safety Data Sheet (SDS) before use. Regulations may vary by country — verify compliance with local authorities.
In conclusion, sourcing potassium periodate requires careful consideration of purity, supplier reliability, regulatory compliance, and intended application. It is essential to obtain the compound from reputable chemical suppliers—whether commercial, industrial, or laboratory-grade—ensuring that quality standards such as ACS or analytical reagent specifications are met when necessary. Factors such as cost, availability, shipping requirements (particularly due to its oxidizing properties), and safety data should also be evaluated. By verifying certifications, reviewing material safety data sheets (MSDS), and considering environmental and handling precautions, organizations can ensure a safe, compliant, and efficient supply chain for potassium periodate. Proper due diligence in sourcing not only supports operational effectiveness but also maintains safety and regulatory adherence in research, industrial, or pharmaceutical applications.