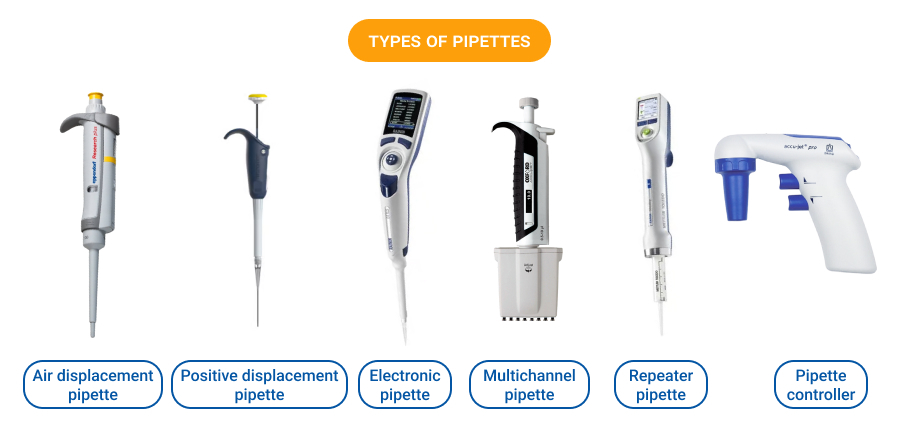
The global pipette market is experiencing steady expansion, driven by increasing demand for precision liquid handling in life sciences, pharmaceuticals, and diagnostics. According to Grand View Research, the global laboratory pipettes market was valued at USD 1.5 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.8% from 2023 to 2030. This growth is fueled by rising R&D investments, automation in laboratories, and stricter regulatory standards requiring high accuracy in sample handling. As demand intensifies, manufacturers of pipette sizing equipment—critical for calibration and quality assurance—are playing an increasingly vital role in ensuring measurement reliability. In this evolving landscape, eight key manufacturers have emerged as leaders, combining innovation, precision engineering, and global reach to support laboratories in maintaining compliance and reproducibility.
Top 8 Pipette Sizes Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Pipettes and Pipette Tips
Domain Est. 1991
Website: bio-rad.com
Key Highlights: Bio-Rad manufactures pipette tips in a range of sizes to fit all our micropipettes and many other manufacturers. To determine tip compatibility with your ……
#2 All Pipettes, Dispensers & Automated Liquid Handlers
Domain Est. 1995
Website: eppendorf.com
Key Highlights: Eppendorf lab pipettes & dispensers are crafted to support you with high accuracy and precision and to allow you to work in an efficient manner….
#3 Pipetting
Domain Est. 1996
Website: sartorius.com
Key Highlights: Our ergonomic pipettes make pipetting easy, accurate and comfortable. See our full range of mechanical and electronic pipettes as well as pipette tips and ……
#4 Your Reliable Partner For Productive Pipettes
Domain Est. 1997
Website: integra-biosciences.com
Key Highlights: Would you like to improve your pipetting efficiency? We provide you with cutting-edge pipettes, facilitating whatever pipetting task you might have….
#5 Single
Domain Est. 1998
Website: hamiltoncompany.com
Key Highlights: $10 deliveryHamilton’s adjustable volume pipettes provide precise, reliable liquid handling with ergonomic design and easy calibration….
#6 Single & Multichannel MLA Pipettes
Domain Est. 1999
Website: vistalab.com
Key Highlights: VistaLab is the innovator behind single & multichannel MLA pipettes. A laboratory tradition for over 4 decades, MLA pipettes are ideal for clinical work….
#7 Pipette.com – your go
Domain Est. 2000
Website: pipette.com
Key Highlights: Pipette.com is your go-to scientific supplies company providing high-quality laboratory consumables and equipment for more than 25 years….
#8 Pipettes and Pipette Tips
Domain Est. 2006
Website: thermofisher.com
Key Highlights: Offering a range of manual and electronic pipettes known for precision, reliability, and ergonomic design….
Expert Sourcing Insights for Pipette Sizes

2026 Market Trends for Pipette Sizes
The global pipette market is poised for significant evolution by 2026, driven by advancements in laboratory automation, increasing demand for precision in life sciences, and the expansion of biopharmaceutical R&D. A key aspect of this transformation is the shifting demand across various pipette sizes, influenced by application-specific needs and technological innovation.
One major trend is the rising preference for smaller volume pipettes (0.1–10 µL). This shift is being fueled by the growing adoption of high-throughput screening (HTS), genomics, and next-generation sequencing (NGS), where minimal reagent use and ultra-precise liquid handling are critical. Microfluidics and single-cell analysis are also pushing demand for low-volume accuracy, making micropipettes increasingly essential in advanced research settings.
Concurrently, mid-range pipettes (10–1000 µL) remain the workhorses of clinical diagnostics and routine laboratory workflows. These sizes continue to dominate in ELISA, PCR setup, and microbiological assays. The market for this segment is expected to grow steadily, with manufacturers focusing on ergonomics, calibration accuracy, and compatibility with automated liquid handling systems.
Larger volume pipettes (1–10 mL) are seeing moderate growth, primarily in environmental testing, quality control, and industrial microbiology. However, their adoption is being tempered by the trend toward miniaturization and reduced sample/reagent volumes. Nonetheless, they remain indispensable in applications requiring bulk liquid transfers, such as media preparation and buffer dilution.
Another notable trend is the integration of electronic pipettes across all size categories. By 2026, smart pipettes with programmable volume settings, Bluetooth connectivity, and cloud-based data logging are expected to gain significant market share. These devices enhance reproducibility and traceability, especially in regulated environments like pharmaceutical manufacturing and clinical labs.
Regionally, North America and Europe lead in the adoption of advanced pipetting technologies, while the Asia-Pacific region is projected to witness the fastest growth due to expanding research infrastructure and increasing investments in biotechnology in countries like China, India, and South Korea.
In summary, the 2026 pipette size market will be characterized by a dual trend: increased demand for precision in low-volume pipettes driven by cutting-edge research, and sustained use of mid-range pipettes in routine diagnostics, supported by technological enhancements across all categories. Manufacturers who innovate in accuracy, connectivity, and user experience are likely to capture the largest market share.

Common Pitfalls When Sourcing Pipette Sizes (Quality, IP)
Sourcing the right pipettes—especially across various sizes—is critical for accuracy and reproducibility in laboratories. However, several common pitfalls can compromise quality, performance, and intellectual property (IP) compliance. Being aware of these risks helps ensure reliable results and regulatory adherence.
Overlooking Calibration and Accuracy Verification
Many labs assume that all pipettes, especially from well-known brands, arrive pre-calibrated to exacting standards. However, variations in manufacturing, shipping, or storage can affect performance. Failing to verify accuracy upon receipt—or at regular intervals—leads to inconsistent results. Always source pipettes from suppliers that provide traceable calibration certificates and support routine recalibration.
Ignoring Material Compatibility and Chemical Resistance
Different pipette sizes may use varied materials for seals, plungers, or body components. Using a pipette with incompatible materials (e.g., certain plastics degrading when exposed to organic solvents) can result in leaks, inaccurate volume delivery, or contamination. Always confirm chemical resistance specifications for each pipette size and application.
Selecting Based on Price Over Total Cost of Ownership
Choosing the cheapest option may seem cost-effective initially but can lead to frequent replacements, calibration failures, or poor ergonomics causing user fatigue and errors. Low-cost pipettes may lack durability or precision, increasing long-term costs. Evaluate total cost of ownership, including maintenance, calibration frequency, and warranty support.
Neglecting Ergonomic Design Across Sizes
Pipettes come in various sizes for different volume ranges, but ergonomic consistency is often overlooked. Users switching between small (e.g., 0.5–10 µL) and large (e.g., 1,000–5,000 µL) volume pipettes may experience strain if designs vary significantly. Poor ergonomics increase the risk of repetitive strain injuries (RSIs) and volume inaccuracy due to inconsistent plunger pressure.
Assuming Universal Tip Compatibility
Not all pipette sizes are compatible with every tip brand, even if they appear to fit. Poor tip sealing can cause leaks, aerosol contamination, or volume inaccuracy. Some manufacturers design pipettes to work optimally only with proprietary tips—raising both performance and IP concerns. Always verify tip compatibility and be cautious of third-party tips that may infringe on patented designs.
Overlooking Intellectual Property (IP) Risks with Third-Party Products
Using generic or third-party pipettes or tips can pose IP risks if they replicate patented technologies (e.g., specific sealing mechanisms, calibration systems, or tip-ejection designs). Infringement may lead to legal action or supply chain disruptions. Source from reputable suppliers that ensure IP compliance and offer indemnification where applicable.
Failing to Validate for Regulated Environments
In GLP, GMP, or ISO-certified labs, every pipette must be validated and documented. Sourcing pipettes without proper documentation, software integration, or audit trails can compromise compliance. Ensure the supplier provides full validation support, including IQ/OQ/PQ documentation tailored to each pipette size.
Underestimating the Need for Volume-Specific Performance Testing
Pipette accuracy can vary significantly across its volume range. A 1,000 µL pipette may be accurate at 1,000 µL but less so at 100 µL. Always request performance data across the full range for each size and verify through independent testing if necessary.
By addressing these common pitfalls, laboratories can ensure they source pipette sizes that deliver consistent quality, maintain regulatory compliance, and avoid unnecessary IP exposure.

Logistics & Compliance Guide for Pipette Sizes
Proper handling, storage, transportation, and regulatory compliance for pipettes—especially when dealing with varying sizes—are essential for maintaining accuracy, traceability, and adherence to laboratory standards. This guide outlines key considerations for managing pipettes of different sizes across logistical and compliance frameworks.
Understanding Pipette Size Classifications
Pipettes are typically categorized by volume range, which determines their use case and handling protocols:
- Microliter (µL) Pipettes: 0.1–1,000 µL (e.g., P10, P100, P1000)
- Milliliter (mL) Pipettes: 1–100 mL (e.g., serological or mechanical pipettes)
- Multichannel Pipettes: 8- or 12-channel variants, often used in high-throughput environments
Different sizes may require unique storage, calibration, and usage documentation, especially in regulated industries such as pharmaceuticals, clinical diagnostics, and environmental testing.
Storage & Handling Requirements
- Environment: Store pipettes vertically in designated racks to prevent contamination and internal mechanism damage. Avoid exposure to extreme temperatures, humidity, and corrosive vapors.
- Separation by Size: Organize pipettes by size and volume range to prevent cross-contamination and misuse. Color-coded racks can aid in quick identification.
- Protective Tips: Always use tip ejectors; never manually remove tips to avoid shaft contamination or mechanical stress.
- Cleanliness: Clean and decontaminate pipettes regularly using manufacturer-recommended procedures, especially when switching between volume ranges or applications (e.g., DNA vs. RNA work).
Transportation Guidelines
- Domestic/Internal Transfers: Use padded containers or pipette carriers to prevent damage during lab-to-lab movement.
- International Shipping:
- Declare pipettes as scientific instruments; no special hazardous classification unless contaminated.
- Use shock-absorbent packaging, especially for adjustable or electronic models.
- Include desiccants if shipping to high-humidity regions.
- Documentation: Maintain shipping logs with serial numbers, sizes, and calibration status for audit trails.
Calibration & Maintenance Compliance
- Frequency: Calibrate pipettes based on use frequency and regulatory requirements:
- High-use: Every 3–6 months
- Standard-use: Annually
- Critical applications (e.g., GLP, GMP): Every 3 months
- Standards: Follow ISO 8655 (International Standard for Piston-Operated Volumetric Apparatus) and manufacturer guidelines.
- Records: Maintain logs specifying pipette size, serial number, calibration date, technician, results (accuracy & precision), and next due date.
- Traceability: Use unique identifiers (barcodes, QR codes) linked to size and calibration history for full auditability.
Regulatory & Industry Compliance
- GLP (Good Laboratory Practice): Requires documented calibration, traceable standards, and training records for all pipette sizes used in non-clinical studies.
- GMP (Good Manufacturing Practice): Pipettes used in production or quality control must be calibrated and validated according to defined SOPs.
- CLIA (Clinical Laboratory Improvement Amendments): Mandates regular calibration and maintenance of all laboratory equipment, including size-specific pipettes.
- ISO 17025: Laboratories seeking accreditation must demonstrate metrological traceability and measurement uncertainty for pipetting volumes.
Training & User Accountability
- Size-Specific Training: Personnel must be trained on proper use, including volume setting, pipetting technique, and tip compatibility for each pipette size.
- Usage Logs: In regulated environments, maintain logs indicating which pipette (by size and ID) was used in specific procedures.
- Error Reporting: Establish protocols for reporting damaged or out-of-tolerance pipettes, with immediate quarantine and recalibration.
Disposal & End-of-Life
- Non-Contaminated Pipettes: Recycle through e-waste programs if electronic; otherwise, dispose per local regulations.
- Biohazard-Exposed Pipettes: Decontaminate following biosafety protocols (e.g., autoclaving) before disposal as biohazard waste.
- Documentation: Record retirement of pipettes, including reason (e.g., failed calibration, damage), size, and disposal method.
Summary
Effective logistics and compliance for pipette sizes require systematic organization, regular calibration, adherence to international standards, and thorough documentation. By aligning storage, transport, maintenance, and training practices with the specific needs of each pipette size, laboratories can ensure data integrity, regulatory compliance, and instrument longevity.
Conclusion for Sourcing Pipette Sizes:
Selecting the appropriate pipette sizes is critical to ensuring accuracy, precision, and efficiency in laboratory workflows. Based on the evaluation of experimental requirements, volume ranges, and throughput needs, it is recommended to source a combination of adjustable and fixed-volume pipettes that cover the full spectrum of volumes typically used—ranging from microliters to milliliters. Prioritizing ergonomic, calibrated, and durable pipettes from reputable suppliers will enhance user comfort, reduce variability, and support compliance with quality standards. Additionally, investing in multichannel pipettes for high-throughput applications can significantly improve productivity. Ultimately, a well-curated pipette inventory tailored to specific lab protocols will optimize experimental outcomes and contribute to consistent, reproducible results.