The global market for microbiological culture media, including specialized products like phenol red broth, is experiencing steady growth driven by rising demand in clinical diagnostics, pharmaceutical quality control, and academic research. According to Mordor Intelligence, the culture media market is projected to grow at a CAGR of approximately 7.2% from 2023 to 2028, fueled by increased R&D spending and stringent regulatory requirements for sterility testing. Phenol red broth, a differential medium widely used for carbohydrate fermentation tests, remains a staple in microbiology laboratories due to its reliability in detecting pH changes associated with microbial metabolism. With increasing investments in biotechnology and infectious disease research, the demand for high-quality, consistent formulations has intensified. As a result, several manufacturers have emerged as leaders in producing ready-to-use and customizable phenol red broth formulations that meet ISO and USP standards. Below are the top five manufacturers recognized for their product quality, scalability, and technical support in the global life sciences supply chain.
Top 5 Phenol Red Broth Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Phenol Red Broth Base
Domain Est. 1990
Website: bd.com
Key Highlights: BD supplies more than 400 different BD Difco™ and BD BBL™ brand media formulations and ingredients in dehydrated form for the convenience of the ……
#2 Phenol Red Broth Base Lactose with Durham Tube, Prepared Media …
Domain Est. 1995
Website: carolina.com
Key Highlights: Designed for use with Escherichia coli and Proteus mirabilis species of bacteria. It is recommended that prepared media tubes be used within 3 months of ……
#3 Phenol Red Broth Base
Domain Est. 2000
#4 Phenol Red Broth Base Formato 500 g
Domain Est. 2001
Website: condalab.com
Key Highlights: Phenol Red Broth Base ; Catalogue number: 1115 ; Applications. General use Confirmation ; Industry. Clinical General cultivation ; Pack size ; Sodium chloride, 5 ……
#5 Phenol Red Broth Base
Domain Est. 2002
Website: cdhfinechemical.com
Key Highlights: Description. a basal medium to which carbohydrates are added for determination of fermentation reactions of pure cultures of microorganisms….
Expert Sourcing Insights for Phenol Red Broth

H2: Market Trends for Phenol Red Broth in 2026
The global market for Phenol Red Broth, a widely used differential microbiological medium, is expected to experience steady growth by 2026, driven by rising demand in clinical diagnostics, pharmaceutical quality control, and academic research. Key market trends shaping the landscape include:
-
Increased Adoption in Clinical Microbiology
Phenol Red Broth continues to be a staple in identifying carbohydrate fermentation patterns of bacteria, particularly in distinguishing enteric pathogens. With rising incidences of gastrointestinal infections and antimicrobial resistance, clinical laboratories are expanding their use of cost-effective, reliable culture media like Phenol Red Broth for preliminary bacterial identification. -
Growth in Biopharmaceutical Manufacturing
The biopharmaceutical sector is increasingly relying on microbiological testing for sterility assurance and contamination control. Phenol Red Broth is often used in media fill simulations and environmental monitoring. As regulatory standards tighten globally—especially under FDA and EMA guidelines—demand for validated culture media, including Phenol Red formulations, is rising. -
Expansion of R&D in Academic and Contract Research Organizations (CROs)
Universities and CROs engaged in microbiology, metabolic studies, and vaccine development are significant consumers of culture media. Increased funding for infectious disease research and microbiome studies is expected to boost demand for specialized broths, including Phenol Red variants tailored to specific fermentation tests. -
Shift Toward Dehydrated and Ready-to-Use Media
A notable trend is the preference for dehydrated powder and ready-to-use liquid formulations of Phenol Red Broth. This shift is driven by standardization needs, reduced preparation time, and minimized contamination risks in high-throughput labs. Major suppliers are investing in pre-sterilized, single-use formats to cater to this demand. -
Regional Market Growth in Asia-Pacific
Emerging economies in Asia-Pacific, particularly India, China, and South Korea, are witnessing rapid expansion in healthcare infrastructure and biotech sectors. Local production of culture media and partnerships with global suppliers are enhancing accessibility, contributing to regional market growth. -
Sustainability and Quality Compliance
Manufacturers are focusing on sustainable sourcing of raw materials and reducing the environmental impact of production. At the same time, adherence to ISO 13485 and other quality management systems is becoming a competitive differentiator. Certifications ensure batch-to-batch consistency and regulatory compliance, especially for export markets. -
Technological Integration and Automation Compatibility
While Phenol Red Broth remains a conventional test medium, integration with automated microbial detection systems is on the rise. Some manufacturers are reformulating broths to be compatible with high-throughput screening platforms, enhancing their utility in modern labs.
In summary, the Phenol Red Broth market in 2026 is characterized by steady demand from healthcare and biopharma sectors, regional diversification, and a push toward product standardization and operational efficiency. Continued innovation in formulation and delivery formats will be key to maintaining relevance in an evolving diagnostic ecosystem.
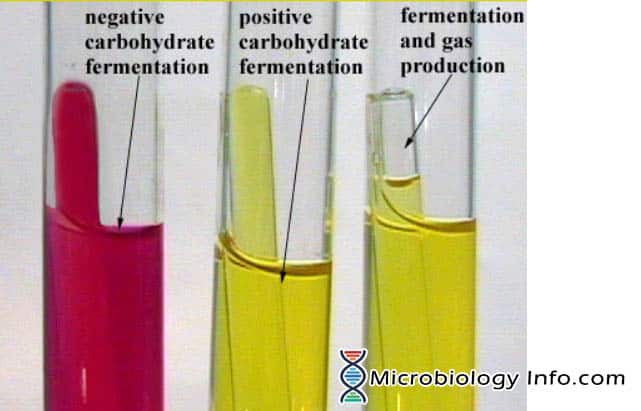
Common Pitfalls Sourcing Phenol Red Broth (Quality, IP)
Sourcing Phenol Red Broth, a critical culture medium used in microbiology for carbohydrate fermentation tests, requires careful attention to both quality and intellectual property (IP) considerations. Overlooking these aspects can lead to unreliable test results, regulatory non-compliance, and potential legal issues.
Quality Pitfalls
- Inconsistent Formulation: Variations in the concentrations of peptone, phenol red indicator, or the specific carbohydrate (e.g., glucose, lactose, sucrose) between batches or suppliers can lead to inconsistent or unreliable fermentation test results. This undermines the reproducibility essential for diagnostic and research applications.
- Contamination: Poor manufacturing practices can result in microbial contamination of the broth, leading to false-positive results (e.g., turbidity or pH change due to contaminant growth, not the target organism’s fermentation).
- Degraded Components: Exposure to light, heat, or moisture during storage or transport can degrade the phenol red indicator or the carbohydrate, reducing sensitivity and accuracy. Old stock may show altered color or fail to produce the expected color change upon fermentation.
- Incorrect pH: The initial pH of the broth is crucial. If it’s not within the specified range (typically around 7.4), the baseline color may be incorrect, making interpretation of acid production (yellow) or gas production (bubble in Durham tube) difficult or inaccurate.
- Lack of Performance Testing: Suppliers that do not provide or perform standardized performance testing (e.g., growth promotion tests with specific control organisms, demonstration of expected fermentation patterns) make it difficult for the end-user to verify the medium’s reliability upon receipt.
Intellectual Property (IP) Pitfalls
- Patented Formulations: Specific formulations of Phenol Red Broth, particularly those optimized for particular applications or incorporating unique additives, may be protected by patents. Sourcing from a manufacturer using such a patented formula without a license could expose the end-user or distributor to infringement claims, especially in commercial diagnostic settings.
- Trademarked Names: While “Phenol Red Broth” is a generic term, specific brand names (e.g., “PRB-Lactose,” “Carbo-Ferm Broth”) used by established suppliers are often trademarked. Using these exact names for your own sourced or in-house prepared product could constitute trademark infringement.
- Proprietary Manufacturing Processes: Even if the formulation is generic, the specific manufacturing process (e.g., a unique method ensuring superior solubility, stability, or reduced background) might be protected as a trade secret. Sourcing from a competitor who reverse-engineers such a process could have legal implications.
- Regulatory Compliance & Labeling: Using a product sourced from a supplier with questionable IP practices might indirectly involve the end-user, particularly if the product is used in regulated environments (e.g., clinical diagnostics, pharmaceuticals). Regulatory bodies may question the legitimacy and traceability of reagents with potential IP issues. Accurate labeling that avoids infringing trademarks is essential.
- Supply Chain Risk: Relying on a supplier whose product is later found to infringe IP can lead to sudden supply disruptions, product recalls, or legal liabilities, disrupting operations and damaging reputation.
Mitigation: To avoid these pitfalls, procure Phenol Red Broth from reputable, established suppliers with transparent quality control (providing CoAs, performance data), clear documentation, and a history of respecting IP. Verify the formulation against standard references (e.g., USP, EP, FDA BAM) and ensure branding and labeling do not infringe on protected trademarks. For critical applications, consider supplier audits and contractual assurances regarding IP indemnification.

Logistics & Compliance Guide for Phenol Red Broth
1. Storage and Handling
Storage Conditions:
– Store Phenol Red Broth powder in a cool, dry place at temperatures between 15–25°C (59–77°F).
– Protect from moisture and light. Keep container tightly closed when not in use.
– Once prepared, sterilized media should be stored at 2–8°C (36–46°F) and used within the validated shelf life (typically 4–6 weeks). Avoid freezing.
– Label all prepared media with preparation date, expiration date, and batch/lot number.
Handling Precautions:
– Use clean, non-reactive equipment (glass or autoclavable plastic) for preparation.
– Always wear appropriate personal protective equipment (PPE), including gloves, lab coat, and safety goggles, during handling.
– Avoid inhalation of powder or aerosol generation during preparation.
– Prepare in a well-ventilated area or under a fume hood if handling large quantities.
2. Preparation and Sterilization
Preparation Instructions:
– Follow the manufacturer’s instructions for reconstitution (typically dissolve in purified water at specified concentration, e.g., 24 g/L).
– Adjust pH to the required range (usually ~7.4 ± 0.2) before sterilization, as specified by the manufacturer.
– Dispense into appropriate containers (e.g., test tubes, bottles) taking care to avoid contamination.
Sterilization:
– Autoclave at 121°C (15 psi) for 15 minutes.
– Do not overheat, as excessive heat may degrade components or alter pH.
– Allow media to cool slowly to prevent precipitation or uneven distribution.
3. Transportation
Domestic and International Shipping:
– Dry Powder: Pack in sealed, moisture-proof containers. Ship as non-hazardous biological substance (UN3373, if applicable, under biological category B). No special temperature control typically required.
– Prepared Media: Transport refrigerated (2–8°C) using validated cold chain packaging. Use insulated containers with ice packs or dry ice if necessary.
– Clearly label packages with contents, storage conditions, and “Perishable – Keep Refrigerated” if applicable.
– Comply with IATA/ICAO regulations for biological substances when shipping internationally.
4. Regulatory Compliance
Classification:
– Phenol Red Broth is generally classified as a non-hazardous chemical when shipped in dry form under normal conditions.
– Under GHS (Globally Harmonized System), review the Safety Data Sheet (SDS) for specific hazard classification. Phenol red may be labeled with:
– H315: Causes skin irritation
– H319: Causes serious eye irritation
– H335: May cause respiratory irritation
– No known carcinogenic or environmental hazards at typical use concentrations.
Documentation:
– Maintain Safety Data Sheets (SDS) readily accessible to all users.
– Ensure product is used in compliance with institutional biosafety and laboratory safety protocols.
– For clinical or diagnostic use, verify compliance with relevant regulatory bodies (e.g., FDA, CE-IVD, CLIA), depending on application.
5. Waste Disposal
- Contaminated Media (with microorganisms): Treat as biohazardous waste. Autoclave before disposal according to local biosafety regulations.
- Unused/Expired Media (uninoculated): May be disposed of as non-hazardous chemical waste in accordance with local environmental regulations. Confirm with institutional EHS (Environmental Health and Safety) guidelines.
- Dry Powder: Dispose of via licensed waste handler if large quantities; otherwise follow institutional chemical waste procedures.
6. Quality Control and Traceability
- Record lot numbers, expiration dates, and preparation logs.
- Perform growth promotion testing using control organisms (e.g., E. coli, S. aureus, Enterococcus faecalis) as part of quality assurance.
- Monitor pH before and after sterilization to ensure consistency.
Note: Always consult the manufacturer’s product insert and SDS for specific handling, safety, and compliance information relevant to your batch and region.
Conclusion for Sourcing Phenol Red Broth:
After evaluating various suppliers and assessing factors such as product quality, consistency, cost, availability, and regulatory compliance, it is concluded that sourcing phenol red broth from reputable life science and microbiology supply companies ensures reliability and performance in laboratory applications. Suppliers that provide sterile, pre-formulated broth with lot traceability and adherence to international quality standards (such as ISO certification) are recommended for consistent and accurate microbiological testing. Establishing a relationship with a trusted vendor not only supports reproducible results in carbohydrate fermentation tests but also streamlines inventory management and reduces preparation time. Therefore, careful selection based on quality assurance, customer support, and timely delivery is essential for effective and efficient laboratory operations.