The global pediatric medical devices market is experiencing steady expansion, driven by increasing healthcare investments, rising awareness of pediatric care, and advancements in child-specific medical instrumentation. According to Mordor Intelligence, the global pediatric medical devices market was valued at USD 28.3 billion in 2023 and is projected to grow at a CAGR of approximately 6.8% from 2024 to 2029. This growth is underpinned by the growing demand for specialized diagnostic tools tailored to children, including pediatric speculums—essential instruments used in otolaryngology, gynecology, and anorectal examinations.
As clinicians emphasize early diagnosis and age-appropriate medical interventions, manufacturers are increasingly focusing on ergonomic designs, improved materials, and size-specific solutions for neonatal, infant, and adolescent patients. With hospitals and outpatient clinics upgrading their diagnostic capabilities, the need for reliable, high-quality pediatric speculums has intensified. This report identifies the top eight manufacturers leading innovation and market presence in this niche, evaluating their product portfolios, regulatory compliance, geographic reach, and technological advancements shaping the future of pediatric diagnostics.
Top 8 Pediatric Speculums Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Speculums Archives
Domain Est. 2011
Website: gynexcorporation.com
Key Highlights: 1-day deliveryInstruments, Speculums Pederson and Graves Stainless Steel Open-Sided Speculum $62.95 · Speculum, Stainless Steel · Select options.Missing: pediatric manufacturer…
#2 Speculums for Medical Procedures
Domain Est. 1994
Website: mms.mckesson.com
Key Highlights: Shop our selection of speculums such as Miltex, BR Surgical, Sklar, and our own private label brand, McKesson Brand….
#3 Nasal Speculum, for children, 13 cm
Domain Est. 1995
Website: karlstorz.com
Key Highlights: Nasal Speculum, for children, 13 cm ; Author, HARTMANN ; Total length, 13 cm ; Field of application, Pediatrics ……
#4 Barraquer Wire Eye Speculum, Pediatric
Domain Est. 1995
Website: wpiinc.com
Key Highlights: In stock $31.29 deliveryThese retractors have wire blades that are 10 mm and the maximum spread is 25 mm. These wire retractors are manufactured from surgical grade stainless steel…
#5 Cook Eye Speculum, Pediatric
Domain Est. 1996
Website: fci-ophthalmics.com
Key Highlights: Cook Eye Speculum, Pediatric … Interested? Request more information about this product. REQUEST INFO ……
#6 Speculums
Domain Est. 1997
Website: keelerusa.com
Key Highlights: 30-day returnsAL0100KL · Barraquer Speculum, Eye with solid blades, pediatric size. $95.50 ; AL0120 SKL · Barraquer Lid Speculum, Adult – AL0120 SKL wire, 14mm square blades….
#7 Speculum Pediatric Adjustable
Domain Est. 2001
Website: surgicalinstruments.com
Key Highlights: Speculum Pediatric Adjustable … For more information request a quote, email us at [email protected], or call us at 800-600-0428 from 7:30am to 5:30pm ……
#8 Paediatric Speculums
Domain Est. 2003
Website: surgitrac.com
Key Highlights: Shop CE-certified paediatric speculums at Surgitrac. High-quality ophthalmic tools for precision care. Order now!…
Expert Sourcing Insights for Pediatric Speculums
H2: Market Trends for Pediatric Speculums in 2026
The global pediatric speculum market is poised for steady growth and transformation by 2026, driven by advancements in medical technology, increasing focus on pediatric healthcare, and rising demand for minimally invasive diagnostic procedures. Several key trends are expected to shape the market dynamics during this period:
-
Technological Innovation and Design Improvements
By 2026, manufacturers are increasingly prioritizing ergonomic and child-friendly designs in pediatric speculums. Innovations such as adjustable sizing, flexible materials, and non-intimidating aesthetics (e.g., colorful, cartoon-themed models) are gaining traction to reduce anxiety in young patients. Additionally, integration with digital imaging systems—such as speculums with built-in cameras and LED lighting—is enhancing diagnostic accuracy and enabling telemedicine applications. -
Expansion of Pediatric Healthcare Infrastructure
Growing investments in pediatric healthcare facilities, especially in emerging economies, are fueling demand for specialized pediatric medical devices. Governments and private healthcare providers are expanding children’s hospitals and outpatient clinics, creating a higher need for pediatric-specific instruments like speculums. This trend is particularly evident in regions such as Asia-Pacific and Latin America. -
Rising Awareness of Early Diagnosis
Increased awareness about early detection of pediatric gynecological and ENT conditions is contributing to more frequent use of pediatric speculums in clinical settings. Educational campaigns and training programs for healthcare professionals are promoting proper use and normalization of pediatric examinations, reducing underdiagnosis and improving patient outcomes. -
Demand for Disposable and Hygienic Solutions
Infection control remains a top priority in pediatric care. As a result, there is a growing shift toward single-use, disposable pediatric speculums to minimize cross-contamination risks. Manufacturers are responding by offering cost-effective, sterile, and eco-friendly disposable options, balancing hygiene with environmental concerns. -
Regulatory Support and Standardization
Regulatory bodies are placing greater emphasis on device safety and efficacy for pediatric populations. By 2026, stricter guidelines and standards for pediatric medical instruments are expected to drive innovation and ensure product reliability. Regulatory incentives for pediatric device development in markets like the U.S. (via the FDA’s Pediatric Device Consortium) are further accelerating market growth. -
Market Consolidation and Strategic Collaborations
The pediatric speculum market is witnessing increased collaboration between medical device companies, research institutions, and pediatric clinics. These partnerships aim to co-develop advanced devices tailored to the anatomical and psychological needs of children. Mergers and acquisitions are also on the rise, enabling companies to expand their product portfolios and geographic reach. -
Telehealth Integration and Remote Diagnostics
With the continued expansion of telemedicine, especially post-pandemic, pediatric speculums compatible with remote diagnostics are becoming more relevant. Devices that allow real-time video transmission during examinations support virtual consultations, improving access to specialized care in rural and underserved areas.
Conclusion
By 2026, the pediatric speculum market will be characterized by innovation, improved patient experience, and expanded access to care. Stakeholders who prioritize child-centered design, infection control, and digital integration are likely to lead the market, meeting the evolving needs of both healthcare providers and young patients worldwide.
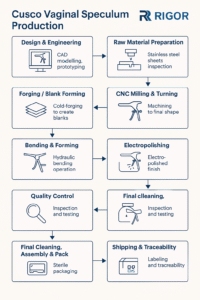
Common Pitfalls When Sourcing Pediatric Speculums: Quality and Intellectual Property Concerns

Logistics & Compliance Guide for Pediatric Speculums
Product Overview and Classification
Pediatric speculums are medical devices designed for the examination of the vaginal canal and cervix in infants, children, and adolescents. These instruments vary in size and material (typically stainless steel or disposable plastic) to accommodate different age groups and clinical needs. They are classified as Class I or Class II medical devices by regulatory bodies such as the U.S. Food and Drug Administration (FDA), depending on design and intended use. Understanding the classification is essential for compliance with manufacturing, importation, and distribution standards.
Regulatory Requirements
Compliance with medical device regulations is mandatory for all stages of the supply chain. In the United States, pediatric speculums must be registered with the FDA under 21 CFR Part 884.4440 (Gynecologic Examination Instrument). Manufacturers and distributors must adhere to Quality System Regulation (QSR) requirements, including design controls, labeling, and recordkeeping. For international markets, compliance with CE marking under the EU Medical Device Regulation (MDR 2017/745) or other regional standards (e.g., Health Canada, TGA in Australia) is required. Ensure all devices have appropriate technical documentation and conformity assessments.
Import and Export Regulations
Importing or exporting pediatric speculums requires adherence to country-specific medical device regulations. In the U.S., the FDA requires prior notification and facility registration for foreign manufacturers. Exporters must provide a Certificate of Free Sale or Certificate to Foreign Government when shipping to certain countries. Additionally, Harmonized System (HS) codes (e.g., 9018.19 for gynecological instruments) must be correctly applied for customs clearance. Be aware of import restrictions, tariffs, and documentation requirements in target markets, including permits from local health authorities.
Labeling and Packaging Standards
Labeling must comply with regulatory standards, including UDI (Unique Device Identification) requirements in the U.S. and EU. Labels should display the device name, model/size, manufacturer information, lot number, expiration date (if applicable), and single-use/disposable warnings where relevant. Packaging must ensure sterility for pre-sterilized items and include instructions for use (IFU) in the local language of the destination country. Mislabeling or incomplete documentation can result in shipment rejection or regulatory penalties.
Storage and Handling
Pediatric speculums, particularly reusable stainless steel models, must be stored in a clean, dry environment to prevent corrosion or contamination. Disposable speculums should be kept in sealed, tamper-evident packaging and stored away from direct sunlight and extreme temperatures. Follow manufacturer guidelines for shelf life and sterilization requirements. Reusable instruments must be cleaned, disinfected, and sterilized according to validated protocols (e.g., autoclaving) before each use.
Distribution and Supply Chain Considerations
Work only with authorized distributors and healthcare providers who comply with medical device handling and inventory management practices. Maintain traceability through the supply chain using inventory tracking systems that support UDI compliance. Ensure cold chain integrity is not required for these devices, but temperature and humidity should still be monitored during transport to prevent packaging damage or material degradation.
Post-Market Surveillance and Reporting
Manufacturers and distributors are responsible for monitoring device performance and reporting adverse events. In the U.S., medical device reporting (MDR) must be submitted to the FDA under 21 CFR Part 803 for incidents involving death, serious injury, or malfunction. The EU requires vigilance reporting under MDR through Eudamed. Establish systems for receiving customer complaints, investigating issues, and initiating recalls if necessary.
Training and Clinical Compliance
Ensure healthcare providers receive proper training on the appropriate selection, use, and sterilization of pediatric speculums. This promotes patient safety and aligns with clinical best practices. Documentation of training may be required for accreditation or regulatory audits. Respect pediatric-specific guidelines from professional organizations such as the American Academy of Pediatrics (AAP) or the Royal College of Paediatrics and Child Health (RCPCH).
Environmental and Disposal Compliance
Dispose of single-use pediatric speculums according to biohazard waste regulations. Reusable instruments must be processed in accordance with clinical sterilization and waste management protocols. Facilities must comply with local environmental regulations for medical waste handling, including segregation, containment, and documentation. Sustainable practices, such as recycling packaging materials, are encouraged where feasible.
Audit and Documentation Readiness
Maintain comprehensive records including device registration, manufacturing certificates, import/export documentation, distribution logs, and post-market surveillance reports. These documents must be available for regulatory audits or inspections. Regular internal audits help ensure ongoing compliance with quality and logistics standards across the product lifecycle.
In conclusion, sourcing pediatric speculums requires careful consideration of several key factors to ensure safety, comfort, and clinical effectiveness. Healthcare providers must prioritize products that are appropriately sized for children, made from high-quality, hypoallergenic materials, and designed with pediatric anatomy in mind. Engaging with reputable suppliers, verifying compliance with regulatory standards (such as FDA or CE marking), and considering feedback from clinical staff are essential steps in the procurement process. Additionally, cost should not be the sole deciding factor—long-term value, durability, and patient comfort must guide purchasing decisions. By thoughtfully selecting pediatric speculums, clinics and hospitals can enhance the patient experience, improve diagnostic accuracy, and uphold the highest standards of pediatric care.