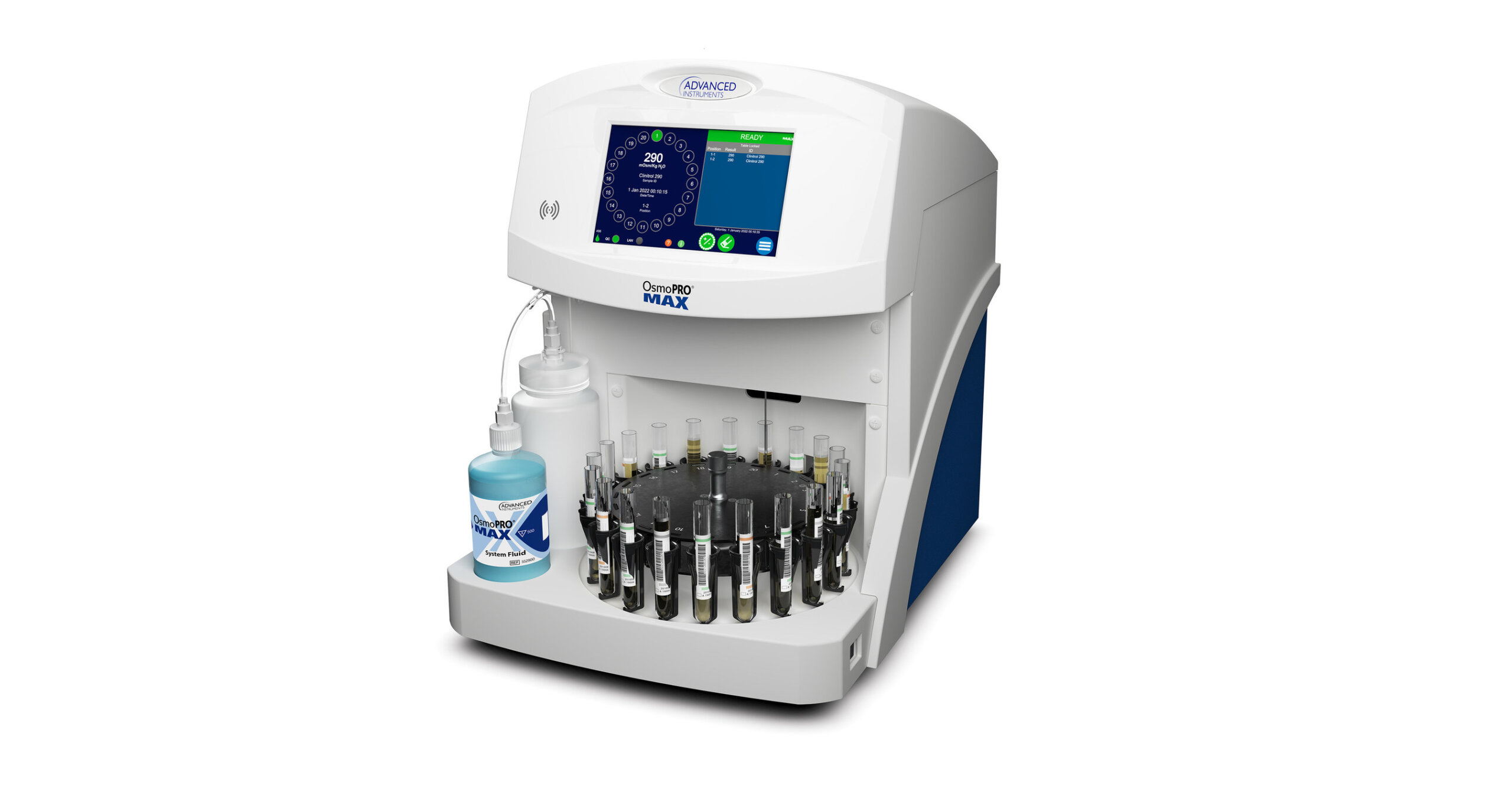
The global osmometer market is experiencing steady growth, driven by increasing demand for quality control in pharmaceuticals, biotechnology, and clinical diagnostics. According to Mordor Intelligence, the Osmometer Market was valued at USD 415.6 million in 2023 and is projected to reach USD 563.8 million by 2029, growing at a CAGR of 5.15% during the forecast period. This expansion is fueled by rising R&D activities in life sciences, stricter regulatory requirements for drug formulation, and the growing emphasis on patient safety in clinical laboratories. As demand for precise and reliable osmolality measurements intensifies, several manufacturers have emerged as key players, consistently innovating to meet evolving industry standards. Below are the top six osmometer manufacturers shaping the market landscape with advanced technology, broad product portfolios, and a strong global presence.
Top 6 Osmometers Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Osmometer
Domain Est. 1999
Website: labtron.com
Key Highlights: Rating 4.7 (630) Labtron Osmometer is specialized for measuring osmotic concentration upto 3000 mOsmol/kg. They feature automatic temperature regulation to adjust measurements …..
#2 Osmometers
Domain Est. 1995
Website: fishersci.com
Key Highlights: Browse a full range of Osmometers products from leading suppliers. Shop now at Fisher Scientific for all of your scientific needs….
#3 Osmometers – Precise Osmometry Solutions
Domain Est. 1998
Website: knauer.net
Key Highlights: KNAUER is one of the pioneers in the field of osmometry and known for its reliable and user-friendly instruments for many decades. View our brochure….
#4 Osmometers
Domain Est. 2000
Website: osmometers.com
Key Highlights: We are the osmometer people, with vapor pressure and freezing point osmometry instruments for all kinds of applications. Osmometers for your Specific Needs….
#5 Advanced Osmometers
Domain Est. 2001
Website: aicompanies.com
Key Highlights: We have a legacy of producing high-quality osmometers that have helped bioprocessing, clinical, food & beverage, and academic labs improve quality….
#6 EN_Homepage
Website: gonotec.de
Key Highlights: Gonotec GmbH is a medium-sized German company which is specialized in the development, manufacture and sale of osmometers for a wide variety of applications….
Expert Sourcing Insights for Osmometers

H2: 2026 Market Trends for Osmometers
The global osmometer market is poised for significant evolution by 2026, driven by advances in medical diagnostics, pharmaceutical quality control, and technological innovation. Osmometers—devices used to measure the osmotic strength or concentration of solute particles in a solution—are critical tools in clinical laboratories, biopharmaceutical manufacturing, and research settings. Several key trends are expected to shape the market landscape in 2026:
1. Rising Demand in Clinical Diagnostics
The increasing prevalence of chronic diseases such as diabetes, kidney disorders, and dehydration-related conditions is fueling demand for accurate and rapid diagnostic tools. Osmometers play a crucial role in assessing urine and serum osmolality, enabling early diagnosis and monitoring of renal function and electrolyte imbalances. As healthcare systems emphasize preventive care and personalized medicine, the integration of osmometry into routine clinical testing is expected to grow.
2. Expansion in Biopharmaceutical and Biotech Applications
The biopharmaceutical industry relies heavily on osmometers to ensure the stability and safety of drug formulations, particularly in parenteral and cell-based therapies. With the surge in monoclonal antibodies, mRNA vaccines, and advanced therapeutics, precise osmolality control during manufacturing and quality assurance has become essential. By 2026, stricter regulatory standards and the need for high-throughput, automated osmometry systems are expected to drive adoption in GMP-compliant facilities.
3. Technological Advancements and Automation
Innovation in osmometer design—such as micro-volume testing, faster measurement times, enhanced accuracy, and connectivity with laboratory information systems (LIS)—is reshaping the market. Automated and semi-automated osmometers are gaining traction, particularly in high-volume laboratories, reducing human error and improving workflow efficiency. Integration with IoT platforms and AI-driven data analytics may further enhance predictive maintenance and result interpretation.
4. Shift Toward Portable and Point-of-Care Devices
There is a growing interest in compact, portable osmometers for use in decentralized settings, including veterinary clinics, field research, and point-of-care diagnostics. These devices offer rapid results with minimal sample volume, making them ideal for emergency departments, sports medicine, and remote healthcare delivery. By 2026, miniaturized osmometers with user-friendly interfaces are expected to gain market share, especially in emerging economies.
5. Regional Market Growth and Regulatory Influence
North America currently dominates the osmometer market due to advanced healthcare infrastructure and high R&D expenditure. However, the Asia-Pacific region is anticipated to register the highest CAGR by 2026, driven by expanding healthcare access, rising biopharmaceutical production in countries like China and India, and increasing investments in clinical laboratories. Regulatory harmonization efforts by agencies such as the FDA and EMA will continue to influence product standards and market entry strategies.
6. Sustainability and Cost Efficiency
As laboratories face budget constraints and environmental concerns, manufacturers are focusing on sustainable design—such as reduced reagent use, longer-lasting sensors, and recyclable components. Cost-effective models with low maintenance requirements are likely to appeal to smaller clinics and resource-limited settings, broadening the market reach.
In summary, the 2026 osmometer market will be characterized by technological innovation, expanding applications in healthcare and biopharma, and growing demand in both developed and emerging regions. Companies that prioritize automation, portability, regulatory compliance, and sustainability are expected to lead the next phase of market growth.

Common Pitfalls Sourcing Osmometers: Quality and Intellectual Property (IP) Concerns
Sourcing osmometers—critical instruments for measuring solute concentration in solutions across clinical, pharmaceutical, and research settings—requires careful evaluation to avoid significant pitfalls related to product quality and intellectual property (IP) risks. Overlooking these aspects can lead to unreliable data, regulatory non-compliance, legal exposure, and reputational damage.
Quality-Related Pitfalls
-
Inadequate Calibration and Traceability
A major quality concern is procuring osmometers without proper calibration documentation or traceability to international standards (e.g., NIST). Instruments lacking certified calibration may produce inaccurate osmolality readings, compromising critical applications such as drug formulation or patient diagnostics. Buyers should verify that suppliers provide valid calibration certificates and support routine recalibration services. -
Poor Build Quality and Reliability
Low-cost osmometers may use substandard materials or components, leading to frequent malfunctions, short service life, or inconsistent performance. For example, poorly manufactured thermistors or sample chambers can introduce measurement drift. It is essential to assess the device’s construction, warranty terms, and user reviews—especially from peer institutions—to gauge long-term reliability. -
Lack of Regulatory Compliance
In regulated environments (e.g., GLP, GMP, or clinical labs), osmometers must comply with standards such as ISO 13485, CE marking, or FDA 21 CFR Part 820. Sourcing instruments from vendors without proper certifications risks non-compliance during audits. Always confirm regulatory status and ensure the device meets jurisdiction-specific requirements. -
Insufficient Validation Support
Many labs require instrument qualification (IQ/OQ/PQ). Vendors that do not provide detailed validation protocols or user manuals create additional burdens for end users. Ensure the supplier offers comprehensive documentation and technical support for validation processes.
Intellectual Property (IP) Pitfalls
-
Risk of Infringing Patented Technology
Osmometry involves proprietary technologies, particularly in freezing point depression and vapor pressure methods. Sourcing from manufacturers that do not respect existing IP rights may expose the buyer to litigation if the device incorporates patented components or methods without authorization. Conduct due diligence on the supplier’s IP position and avoid vendors with questionable reputations or lack of transparency. -
Unclear Ownership in Custom or Modified Devices
When ordering customized osmometers (e.g., for integration into larger systems), ambiguity in IP ownership can arise. Contracts should explicitly define who owns the design modifications, software algorithms, or firmware enhancements. Without clear agreements, buyers may lose control over future use or updates. -
Use of Open-Source or Unlicensed Software
Some osmometers rely on embedded software for data processing and user interface. If the software includes unlicensed or improperly attributed open-source code, it may violate licensing terms (e.g., GPL), leading to legal risks or forced disclosure of proprietary information. Verify the software compliance status with the vendor. -
Counterfeit or Clone Instruments
Particularly in emerging markets, counterfeit or cloned osmometers may imitate reputable brands but lack performance and IP legitimacy. These devices often infringe design patents and trademarks, and their use can expose institutions to legal liability. Purchase only through authorized distributors and verify product authenticity.
Mitigation Strategies
- Conduct Supplier Audits: Evaluate manufacturers’ quality management systems and IP practices before procurement.
- Require Documentation: Insist on calibration certificates, regulatory compliance statements, and IP indemnification clauses in contracts.
- Engage Legal and Technical Experts: Involve IP counsel and lab engineers during the sourcing process to assess risks.
- Prioritize Reputable Vendors: Choose established suppliers with proven track records in scientific instrumentation.
By proactively addressing quality and IP concerns, organizations can ensure the acquisition of reliable, compliant, and legally sound osmometry solutions.

H2: Logistics & Compliance Guide for Osmometers
Proper logistics and compliance management are essential for the safe transportation, installation, operation, and maintenance of osmometers—precision instruments used to measure solute concentration in biological and chemical solutions. This guide outlines key considerations to ensure regulatory adherence, instrument integrity, and operational efficiency.
H2: Regulatory Compliance
1. Medical Device Regulations (if applicable):
– Osmometers used in clinical diagnostics or patient care may be classified as medical devices under regulations such as:
– FDA (U.S.): Ensure 510(k) clearance or premarket approval (PMA) if applicable. Register the device and facility with the FDA.
– EU MDR (EU): Comply with Regulation (EU) 2017/745. Obtain CE marking through a Notified Body if the device falls under Class I (measuring function) or higher.
– Other Regions: Confirm compliance with local regulations (e.g., Health Canada, TGA in Australia, PMDA in Japan).
2. In Vitro Diagnostic (IVD) Directives:
– If used for diagnostic purposes, adhere to IVD-specific requirements, including performance evaluation, risk management (ISO 14971), and labeling per IVD directives.
3. Electrical Safety & EMC:
– Comply with IEC 61010-1 (safety requirements for electrical equipment) and IEC 61326-1 (EMC for laboratory equipment).
– Ensure all osmometers meet local electrical standards (e.g., UL, CSA, CE marking for EMC).
4. Environmental Regulations:
– Follow RoHS and REACH (EU) for hazardous substance restrictions.
– Comply with WEEE directives for proper end-of-life disposal.
H2: Transportation & Handling
1. Shipping Requirements:
– Use manufacturer-approved packaging with shock-absorbing materials to protect sensitive components (e.g., thermistor, sample chamber).
– Maintain ambient temperature during transit; avoid exposure to extreme heat (>40°C) or freezing.
– Include desiccants if shipping to humid environments to prevent condensation.
2. Import/Export Documentation:
– Prepare commercial invoice, packing list, bill of lading, and certificate of origin.
– For international shipments, verify:
– Export Control Classification Number (ECCN) – Most osmometers fall under EAR99 (no license required), but verify with manufacturer.
– Import Permits – Some countries require permits for medical or laboratory equipment.
– Customs Classification (HS Code) – Typically 9027.80 (instruments for measuring or checking quantities of liquids).
3. Cold Chain (if applicable):
– If shipping with temperature-sensitive reagents or calibration standards, use validated cold chain logistics (2–8°C or as specified).
H2: Installation & Site Preparation
1. Environmental Conditions:
– Install in a stable environment with:
– Temperature: 15–30°C
– Humidity: 30–70% (non-condensing)
– Vibration-free, level surface
– Away from direct sunlight and HVAC vents
2. Power Supply:
– Ensure proper voltage (100–240 VAC, 50/60 Hz) and grounding.
– Use surge protectors to prevent electrical damage.
3. Calibration & Qualification:
– Perform initial calibration using certified standards (e.g., aqueous NaCl solutions).
– Document Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) per GxP or ISO 17025 requirements (if used in regulated testing).
H2: Operational & Maintenance Compliance
1. Regular Calibration & Verification:
– Schedule routine calibration per manufacturer’s recommendations (e.g., monthly, quarterly).
– Maintain a traceable calibration log with standards used and due dates.
2. Preventive Maintenance:
– Follow manufacturer’s maintenance schedule (e.g., cleaning sample chambers, inspecting sensors).
– Use only approved spare parts and consumables.
3. Data Integrity (if applicable):
– For regulated environments (e.g., GLP, GMP), ensure:
– Electronic records comply with 21 CFR Part 11 (U.S.) or EU Annex 11.
– Audit trails, user access controls, and data backup procedures are in place.
4. Training & Documentation:
– Train personnel on SOPs for operation, calibration, and troubleshooting.
– Maintain records of training, maintenance, and service.
H2: Disposal & End-of-Life
1. Decommissioning:
– Decontaminate the instrument per biosafety protocols if used with biological samples.
– Remove and properly dispose of any batteries or hazardous components.
2. Recycling & Waste Management:
– Partner with certified e-waste recyclers compliant with WEEE or local regulations.
– Dispose of electronic boards, plastics, and metals according to environmental standards.
3. Data Security:
– If the osmometer stores data, ensure secure data wiping before disposal.
Summary:
Adhering to logistics and compliance protocols ensures the accuracy, safety, and regulatory acceptability of osmometer use. Always consult the manufacturer’s instructions, local regulations, and institutional policies when managing osmometers throughout their lifecycle.
Conclusion for Sourcing Osmometers
In conclusion, sourcing osmometers requires a strategic evaluation of several key factors to ensure the selected instrument meets the specific needs of the intended application, whether in clinical diagnostics, pharmaceutical development, research laboratories, or quality control. Critical considerations include measurement accuracy, sample volume requirements, ease of use, throughput capacity, maintenance needs, and compliance with regulatory standards such as ISO or GLP.
When selecting a supplier, reliability, post-purchase support, calibration services, and cost-effectiveness are equally important. It is recommended to source osmometers from reputable manufacturers or certified distributors with a proven track record in the field of laboratory instrumentation. Additionally, future-proofing through scalable technology, instrument compatibility with existing systems, and potential for software integration can enhance long-term value.
Ultimately, a well-informed sourcing decision—based on technical specifications, vendor reputation, total cost of ownership, and application-specific requirements—will ensure optimal performance, reliability, and compliance, supporting high-quality results across scientific and clinical practices.