The global demand for rapid and accurate diagnostic solutions has surged in recent years, driven by increasing awareness of infectious disease outbreaks and the need for point-of-care testing. The norovirus test kit market is no exception, with strong growth fueled by rising gastroenteritis cases and heightened focus on food safety and public health surveillance. According to Mordor Intelligence, the infectious disease diagnostics market—under which norovirus testing falls—is projected to grow at a CAGR of over 6.5% from 2023 to 2028, with gastrointestinal pathogen testing contributing significantly to this expansion. Similarly, Grand View Research reported that the global gastrointestinal diagnostics market was valued at USD 5.2 billion in 2022 and is expected to grow at a CAGR of 7.1% through 2030. As norovirus remains a leading cause of acute gastroenteritis worldwide, especially in healthcare, food service, and travel sectors, the need for reliable and efficient test kits has never been greater. This growing demand has spurred innovation and competition among manufacturers, resulting in a dynamic landscape of companies delivering high-performance norovirus detection solutions. Below are the top 9 norovirus test kit manufacturers leading the industry through technological advancement, regulatory compliance, and global market reach.
Top 9 Norovirus Test Kit Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
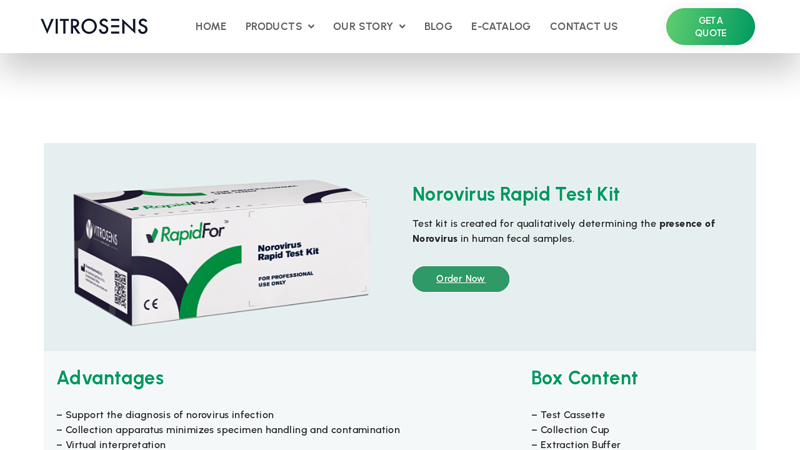
#1 Norovirus Rapid Test Kit
Domain Est. 2020
Website: vitrosens.com
Key Highlights: RapidFor Norovirus Rapid Test Kit qualitatively detects presence of Norovirus in human fecal samples….
#2 Norovirus Testing
Domain Est. 1995
Website: fishersci.com
Key Highlights: Browse a full range of Norovirus Testing products from leading suppliers. Shop now at Fisher Scientific for all of your scientific needs….
#3 Vivalytic
Domain Est. 1997
Website: randox.com
Key Highlights: Vivalytic Norovirus PCR test allows fast and accurate diagnosis of Norovirus infection….
#4 Testing Solutions for Norovirus Detection
Domain Est. 2002
Website: hygiena.com
Key Highlights: Real-time RT-PCR kit for detecting Norovirus GI & GII in food, water & stool samples. ISO/TS 15216 compliant with high sensitivity and built-in process control….
#5 InviScreen® Norovirus Detection Kit
Domain Est. 2004
Website: invitek.com
Key Highlights: InviScreen Norovirus Detection Kit provides a real-time RT-PCR method for the qualitative detection of norovirus genogroups I (GI) and II (GII) in food samples….
#6 Gold Standard Diagnostics
Domain Est. 2006
Website: goldstandarddiagnostics.com
Key Highlights: Global Provider of Diagnostic Test Kits and Instruments. Gold Standard Diagnostics is a global network of companies with decades of experience on the test kit, ……
#7 Bioline Norovirus
Domain Est. 2020
Website: globalpointofcare.abbott
Key Highlights: Bioline Norovirus rapid test is a rapid immunochromatographic assay for qualitative detection of the presence of norovirus antigen….
#8 Norovirus adenovirus and rotavirus rapid test detection
Domain Est. 2022
Website: operondx.com
Key Highlights: Rapid tests for the detection of Rotavirus, Adenovirus and Norovirus genogroup I and II antigens in human faeces in independent bands….
#9 Norovirus – Certest Biotec – Raw Materials
Website: certest.es
Key Highlights: CerTest Norovirus card test offers a simple and a highly sensitive screening assay to make a presumptive diagnosis of Norovirus infection. Specifications….
Expert Sourcing Insights for Norovirus Test Kit
H2: Market Trends in the Norovirus Test Kit Industry for 2026
By 2026, the global norovirus test kit market is expected to experience significant growth, driven by heightened public health awareness, advancements in diagnostic technologies, and increased demand across healthcare and community settings. Key trends shaping the market include:
-
Rising Incidence of Gastrointestinal Infections
Emerging epidemiological data indicate continued outbreaks of norovirus in both developed and developing regions, particularly in healthcare facilities, schools, and cruise ships. This escalating burden of illness is prompting governments and healthcare institutions to invest in rapid and accurate diagnostic tools, fueling demand for norovirus test kits. -
Adoption of Point-of-Care (POC) Testing
There is a growing shift toward decentralized testing models, with increased deployment of rapid antigen detection kits at point-of-care settings. These kits enable faster diagnosis, support immediate infection control measures, and reduce hospitalization duration. By 2026, POC test kits are projected to capture a larger market share due to their ease of use, minimal infrastructure requirements, and quick turnaround time. -
Technological Innovations
Advancements in molecular diagnostics, such as loop-mediated isothermal amplification (LAMP) and multiplex PCR assays, are enhancing the sensitivity and specificity of norovirus detection. Manufacturers are integrating these technologies into automated platforms, allowing high-throughput screening in laboratories. Additionally, digital connectivity features—such as smartphone-linked results and cloud-based data reporting—are being incorporated to improve surveillance and outbreak tracking. -
Expansion in Emerging Markets
Asia-Pacific and Latin America are expected to witness the fastest market growth due to improving healthcare infrastructure, rising government funding for infectious disease control, and increasing awareness of food and water safety. Regulatory reforms and public-private partnerships are facilitating broader access to diagnostic kits in these regions. -
Integration with Food Safety and Public Health Programs
Norovirus test kits are increasingly being adopted beyond clinical settings, including in food processing, water quality monitoring, and outbreak investigation units. Regulatory agencies are mandating more rigorous testing protocols, especially in the food service and hospitality industries, further expanding market opportunities. -
Impact of Pandemic-Era Diagnostic Infrastructure
The infrastructure and supply chains developed during the COVID-19 pandemic have created a favorable environment for rapid scaling of viral diagnostic testing. Many manufacturers have repurposed production lines and distribution networks to include enteric virus testing, supporting faster market entry and cost efficiencies for norovirus test kits. -
Focus on Multiplex Panels
There is a rising preference for multiplex gastrointestinal panels that simultaneously detect norovirus along with other pathogens such as rotavirus, adenovirus, and Salmonella. These comprehensive panels improve diagnostic accuracy and are increasingly adopted in hospital laboratories, contributing to market expansion.
In conclusion, the norovirus test kit market in 2026 will be characterized by technological sophistication, broader geographic reach, and integration into public health and safety systems. As prevention and early detection become central to global health strategies, the demand for reliable, accessible, and rapid diagnostic solutions will continue to rise.

Common Pitfalls Sourcing Norovirus Test Kits: Quality and Intellectual Property Risks
Sourcing Norovirus test kits, especially from international suppliers or emerging manufacturers, presents significant challenges beyond simple procurement. Two critical areas where organizations frequently encounter problems are product quality and intellectual property (IP) infringement. Failing to address these pitfalls can lead to unreliable testing, regulatory non-compliance, financial losses, and reputational damage.
Quality-Related Pitfalls
-
Inaccurate or Unreliable Test Performance
Many low-cost or uncertified kits on the market suffer from poor sensitivity (failing to detect true positive cases) or specificity (producing false positive results). This can lead to missed outbreaks, unnecessary panic, or inappropriate public health responses. Relying on kits without robust clinical validation data significantly compromises diagnostic reliability. -
Lack of Regulatory Compliance
A major risk is sourcing kits that do not meet regulatory standards such as FDA 510(k) clearance, CE-IVDR certification, or other regional requirements. Using non-compliant kits in clinical or public health settings can result in legal liability, rejection by health authorities, and invalid test results that cannot be used for official reporting. -
Inconsistent Manufacturing Standards
Suppliers without adherence to ISO 13485 or Good Manufacturing Practice (GMP) may produce kits with batch-to-batch variability. This inconsistency affects reagent stability, shelf life, and overall test performance, undermining confidence in results over time. -
Inadequate Storage and Shipping Conditions
Norovirus test kits often contain biological components sensitive to temperature and humidity. Poor logistics—such as lack of cold chain management—can degrade kit components before they reach end users, rendering them ineffective even if initially high quality.
Intellectual Property-Related Pitfalls
-
Sourcing Counterfeit or Cloned Kits
The high demand for rapid diagnostics has led to a surge in counterfeit test kits that mimic established brands. These clones may copy patented technologies, designs, or trademarks without authorization, exposing buyers to legal risks and supporting illegal market activity. -
Use of Patented Technology Without Licensing
Many reliable Norovirus tests rely on proprietary assay designs, antibody formulations, or detection methods protected by patents. Sourcing kits from manufacturers that use such IP without proper licensing infringes on patent rights. Buyers may inadvertently become entangled in IP disputes or face injunctions against kit usage. -
Unclear or Missing IP Documentation
Suppliers, particularly smaller or offshore manufacturers, often fail to provide transparent documentation regarding the IP status of their products. The absence of freedom-to-operate (FTO) opinions or licensing agreements increases the risk that the kits violate third-party patents, potentially leading to costly litigation. -
Reputational and Partnership Risks
Partnering with suppliers involved in IP infringement can damage an organization’s reputation, especially in public health or research contexts. It may also jeopardize collaborations with academic institutions or government agencies that require strict adherence to ethical and legal standards.
By proactively evaluating both quality assurance processes and intellectual property integrity, organizations can mitigate these risks and ensure the safe, effective, and legally sound use of Norovirus test kits.
Logistics & Compliance Guide for Norovirus Test Kit
Storage and Handling
Store the Norovirus Test Kit at a controlled temperature between 2°C and 30°C (36°F to 86°F). Avoid exposure to direct sunlight, moisture, and excessive heat. Keep the test kits in their original packaging until ready for use to protect from humidity and contamination. Do not freeze the kit components. Ensure storage areas are clean, dry, and regularly monitored for temperature compliance using calibrated monitoring devices.
Transportation
Transport the Norovirus Test Kit under ambient conditions within the specified temperature range (2°C to 30°C). Use insulated containers with temperature monitoring indicators (e.g., time-temperature indicators or data loggers) when shipping over extended periods or in extreme climates. Shipments should comply with applicable ground and air transport regulations, including IATA Dangerous Goods Regulations if biological samples are included (note: test kits without samples are generally not classified as dangerous goods). Ensure packaging is robust and leak-proof to prevent damage during transit.
Shelf Life and Expiry
The Norovirus Test Kit has a shelf life of 18 months from the date of manufacture, as indicated on the kit label and packaging. Do not use kits beyond the expiration date printed on the box. Monitor inventory using a first-expiry, first-out (FEFO) system to prevent expired use. Document batch numbers and expiration dates upon receipt for traceability.
Regulatory Compliance
The Norovirus Test Kit is CE marked in accordance with Regulation (EU) 2017/746 on In Vitro Diagnostic Medical Devices (IVDR) and is classified as a self-certified Class D IVD device. In the United States, the kit is cleared by the FDA under 510(k) for professional use in clinical laboratories. Ensure local regulatory requirements are met prior to distribution or use, including registration with national competent authorities (e.g., MHRA in the UK, BfArM in Germany). Labeling must include the CE mark, Unique Device Identifier (UDI), manufacturer details, and intended use statement.
Import and Export Documentation
For international shipment, provide a Commercial Invoice, Packing List, Certificate of Conformity, and, where required, an Export Permit or Certificate of Free Sale. Verify import regulations in the destination country—some may require prior approval from health authorities or local representation. For shipments containing biological materials (e.g., control specimens), additional documentation such as a Material Safety Data Sheet (MSDS) and Shipper’s Declaration for Dangerous Goods may be required.
User Training and Documentation
Only trained personnel should perform testing. Provide users with the Instructions for Use (IFU), which include assay principles, procedural steps, interpretation of results, limitations, and safety precautions. Maintain records of user training, test performance, and equipment calibration where applicable. Report any adverse events or device malfunctions to the manufacturer and relevant regulatory bodies per local requirements.
Waste Disposal
Dispose of used test kits and associated materials (e.g., swabs, pipette tips, buffers) in accordance with local biohazard and medical waste regulations. Contaminated materials should be treated as biohazardous waste and autoclaved or incinerated appropriately. Follow OSHA Bloodborne Pathogens Standard (29 CFR 1910.1030) in the U.S. or equivalent guidelines internationally.
Conclusion for Sourcing Norovirus Test Kit:
In conclusion, sourcing a reliable and effective norovirus test kit is essential for ensuring rapid diagnosis, effective outbreak management, and the prevention of disease transmission in clinical, food safety, and public health settings. After evaluating available options, it is critical to prioritize test kits that demonstrate high sensitivity, specificity, regulatory approval (such as FDA clearance or CE marking), ease of use, and fast turnaround time. Additionally, considerations such as cost-effectiveness, storage requirements, and compatibility with existing laboratory infrastructure should inform procurement decisions.
Partnering with reputable manufacturers and suppliers that provide technical support, validation data, and consistent supply chain reliability will enhance testing capabilities and ensure sustained readiness. Ultimately, investing in high-quality norovirus test kits not only supports accurate detection but also strengthens overall public health response, particularly during seasonal outbreaks or in high-risk environments such as healthcare facilities, cruise ships, and food production centers. A strategic and well-informed sourcing approach will maximize the impact of these diagnostic tools in controlling norovirus spread.