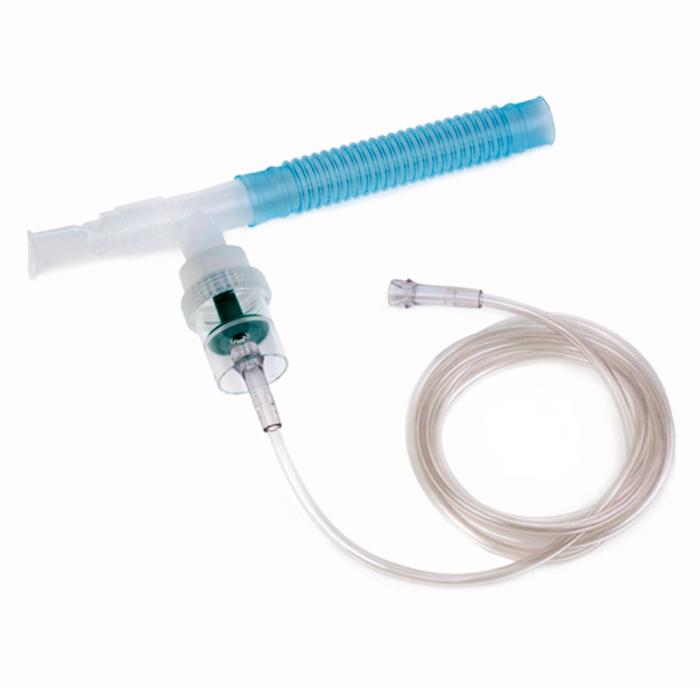
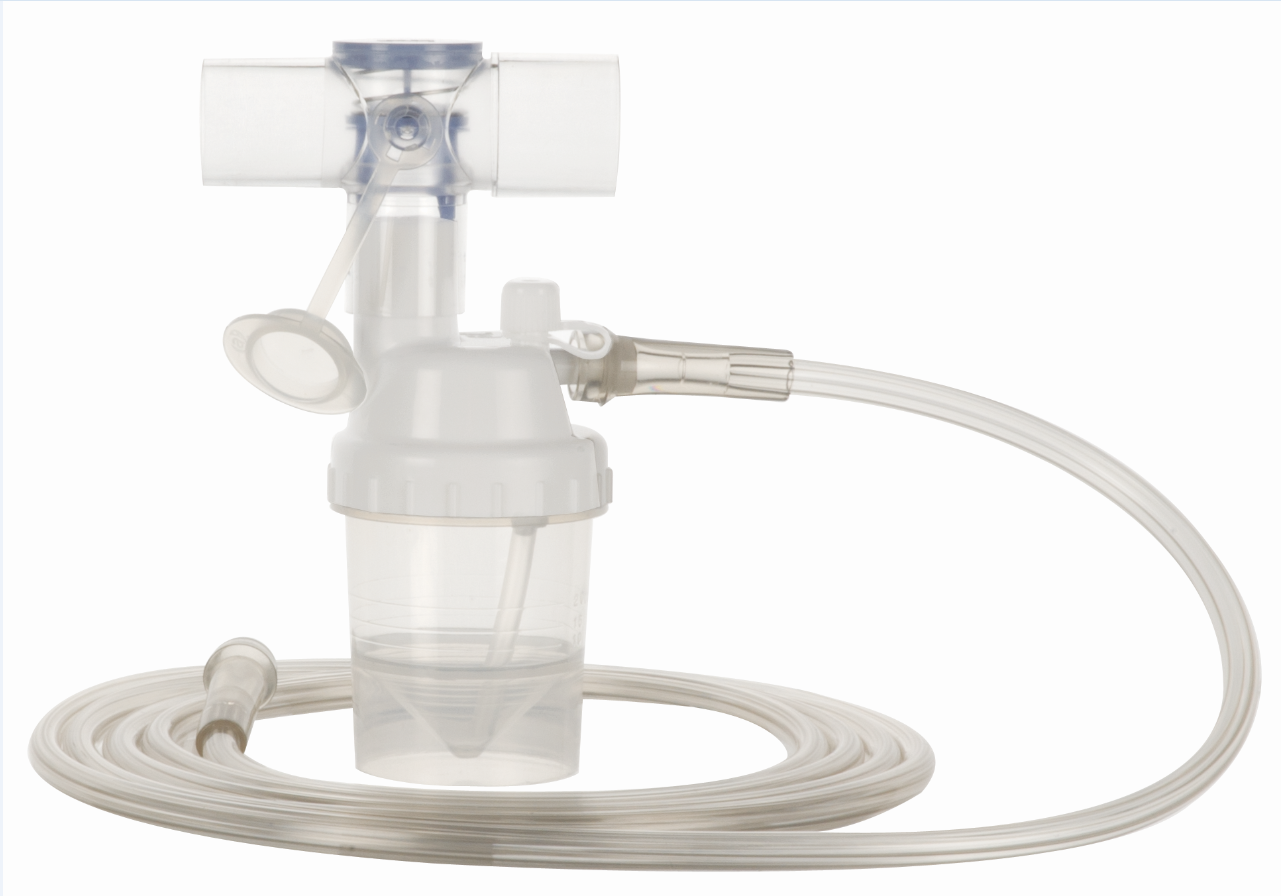
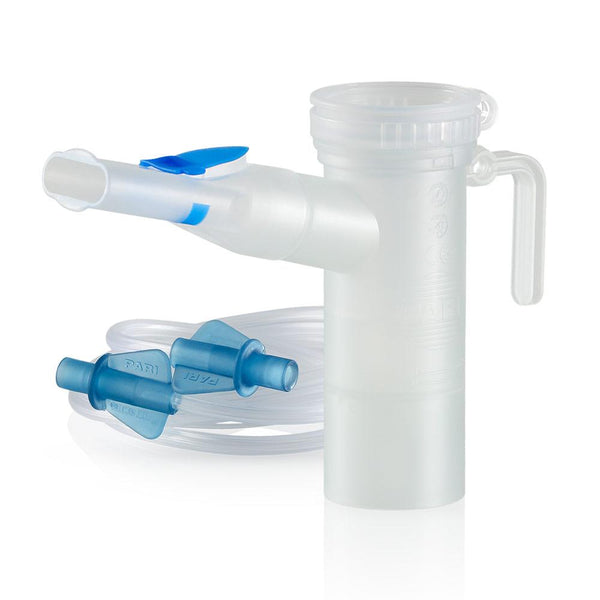
The global nebulizer market is experiencing steady growth, driven by rising prevalence of respiratory disorders such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis. According to a report by Mordor Intelligence, the global nebulizer market was valued at USD 2.8 billion in 2023 and is projected to grow at a CAGR of 7.2% from 2024 to 2029. Similarly, Grand View Research estimates that the market size reached USD 3.1 billion in 2022 and is expected to expand at a CAGR of 7.4% over the same forecast period. This growth trajectory is fueled by increasing home healthcare adoption, technological advancements in nebulization systems, and rising demand for efficient drug delivery devices. As demand surges, nebulisation kit manufacturers are playing a pivotal role in ensuring reliable, hygienic, and compatible components for effective aerosol therapy. Below, we profile the top 8 manufacturers shaping the nebulisation kit landscape through innovation, quality, and global reach.
Top 8 Nebulisation Kit Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Wellead Medical
Domain Est. 2017
Website: wellead.com
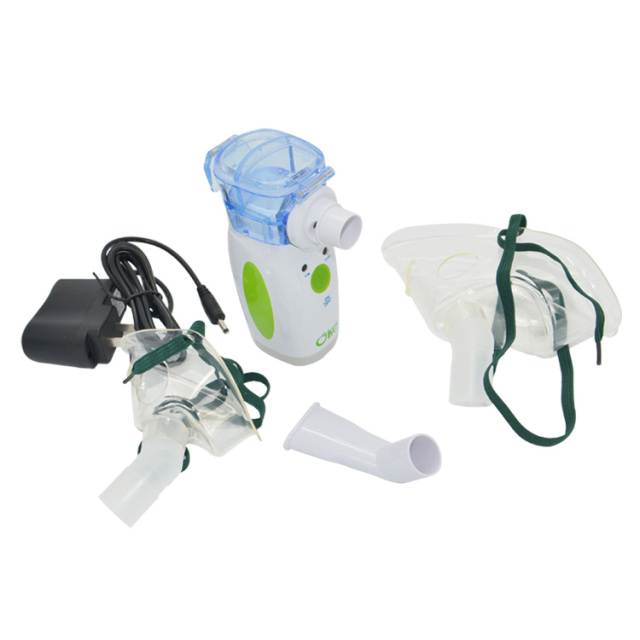
Key Highlights: As a prominent nebulizer mask manufacturer, Wellead Medical designs nebulizer kits that boast an impressive atomization rate of ≥0.2 ml/min….
#2 Asthma management
Domain Est. 1987
Website: usa.philips.com
Key Highlights: A nebulizer changes medication from a liquid to a mist so that it can be more easily inhaled into the lungs….
#3 OMRON Respiratory Nebulizers & Devices for At
Domain Est. 1997
Website: omronhealthcare.com
Key Highlights: OMRON offers prescription-grade, at-home and portable nebulizer solutions to fit a variety of needs and budgets….
#4 Respiratory
Domain Est. 1997
Website: apria.com
Key Highlights: See Apria’s nebulizer products and get support through downloadable manuals….
#5 LC PLUS® Reusable Nebulizer
Domain Est. 1998
Website: pari.com
Key Highlights: Considered the “GOLD Standard” in nebulizer therapy for the treatment of diseases such as asthma, COPD and Cystic Fibrosis….
#6 GaleMed Nebulizers
Domain Est. 1999
Website: galemed.com
Key Highlights: GaleMed offers nebulizers that excel in aerosol delivery for effective respiratory treatment. Discover our solutions for adults, children, and infants, ……
#7 TYVASO® (treprostinil) Nebulizer
Domain Est. 2008
Website: tyvaso.com
Key Highlights: The TYVASO® (treprostinil) Inhalation System is a lightweight, handheld, portable nebulizer. It allows for dosing at home or on the go….
#8 PARI Authorized Nebulizer Machines & Supplies
Domain Est. 2012
Website: nebology.com
Key Highlights: Shop for PARI nebulizer systems, nebulizer cups, replacement parts, and accessories. Clinically proven, and available for shipment direct to your home….
Expert Sourcing Insights for Nebulisation Kit
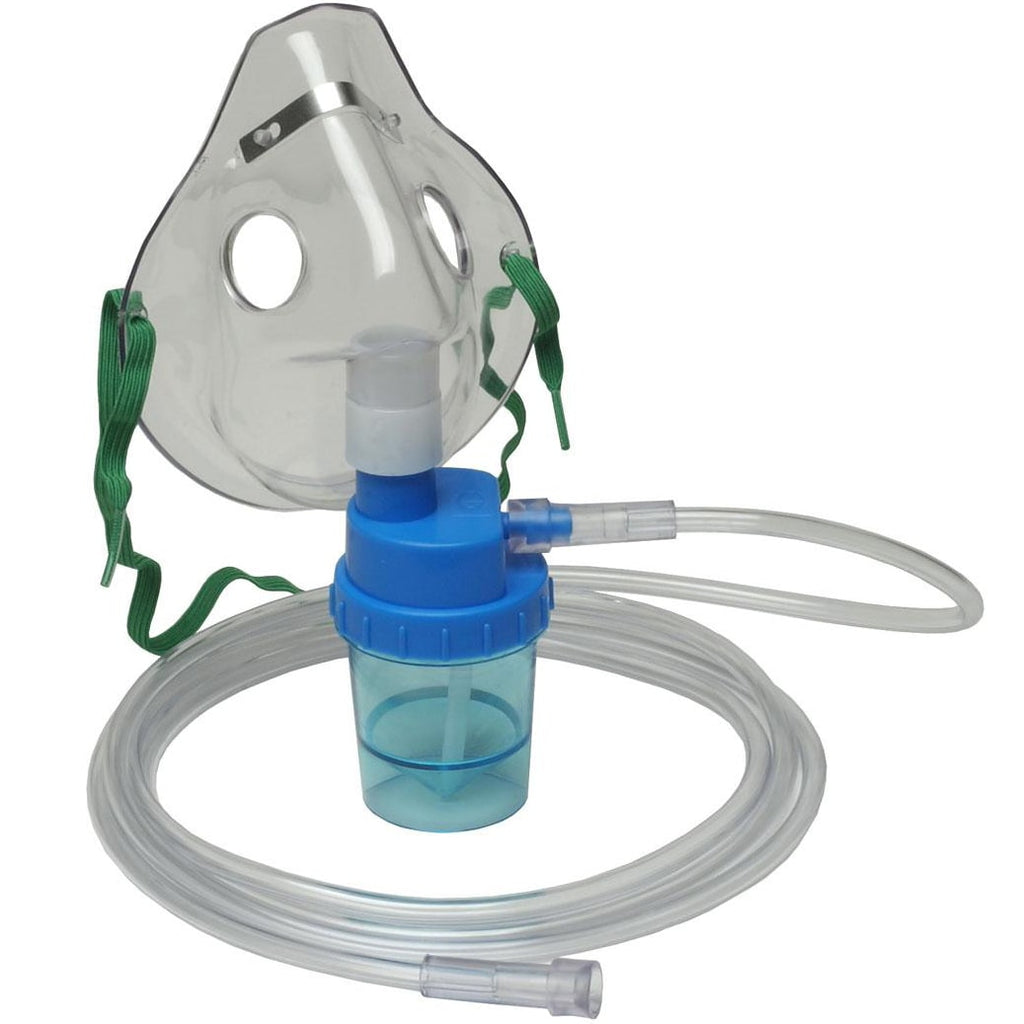
H2: Projected 2026 Market Trends for Nebulisation Kits
The global nebulisation kit market is poised for significant evolution by 2026, driven by a conformation of healthcare demands, technological advancements, and shifting patient preferences. Key trends shaping the market include:
1. Rising Prevalence of Respiratory Diseases: The primary driver remains the increasing global burden of chronic respiratory conditions like asthma, COPD, cystic fibrosis, and the lingering impact of post-COVID respiratory complications. An aging population, particularly in developed economies, further amplifies this demand, ensuring sustained market growth for nebulisation therapy as a crucial delivery method.
2. Dominance of Home Healthcare & Self-Management: A major shift is the migration from hospital/clinical settings to home-based care. Patients and caregivers increasingly prefer the convenience, comfort, and cost-effectiveness of managing respiratory conditions at home. This trend fuels demand for user-friendly, portable, and easy-to-clean nebulisation kits suitable for domestic use, alongside telehealth integration for remote monitoring.
3. Technological Innovation & Product Differentiation: The market is moving beyond basic functionality. Key innovations include:
* Enhanced Portability: Development of ultra-compact, battery-powered, and even wearable nebulisers for greater patient mobility.
* Improved Efficiency & Speed: Adoption of advanced nebuliser technologies (e.g., vibrating mesh, adaptive aerosol delivery) that offer faster treatment times, higher drug delivery efficiency, and quieter operation compared to traditional jet nebulisers.
* Smart Features: Integration of connectivity (Bluetooth, apps) for dosage tracking, treatment adherence monitoring, remote data sharing with healthcare providers, and usage reminders, enhancing patient compliance and outcomes.
* Ergonomic & Child-Friendly Designs: Focus on intuitive interfaces, comfortable masks (including fun designs for children), and simplified cleaning procedures.
4. Growth of the Disposable Kit Segment: Single-use nebuliser kits (cups, tubing, masks) are gaining significant traction, particularly in home settings. This is driven by heightened awareness of infection control (reducing cross-contamination risks), convenience (eliminating complex cleaning), and patient preference, outweighing slightly higher per-use costs for many users. This segment is expected to see substantial growth.
5. Focus on Sustainability & Reusability (Counter-Trend): Alongside disposables, there’s a growing counter-trend driven by environmental concerns and cost pressures. This is spurring innovation in durable, easily cleanable reusable kits made from more sustainable materials and designs that facilitate thorough disinfection, appealing to environmentally conscious consumers and cost-sensitive healthcare systems.
6. Emerging Market Expansion: Rapidly growing healthcare infrastructure, rising disposable incomes, and increasing awareness of respiratory health in regions like Asia-Pacific (especially India and China), Latin America, and parts of Africa present significant growth opportunities. Market players are adapting products (e.g., lower-cost models, simplified designs) and distribution strategies for these regions.
7. Stringent Regulatory Scrutiny & Quality Focus: Regulatory bodies (FDA, EMA, etc.) are placing greater emphasis on device performance, biocompatibility, and accurate drug delivery. This drives manufacturers towards higher quality standards, rigorous testing, and clear documentation, potentially consolidating the market towards established players with robust R&D capabilities.
8. Competitive Landscape & Strategic Moves: The market features established medical device giants and agile niche players. Competition is intensifying, leading to strategic partnerships (e.g., device companies partnering with pharma for combination products), mergers and acquisitions, and a focus on brand differentiation through technology, service, and patient support programs.
In conclusion, the 2026 nebulisation kit market will be characterized by a dynamic interplay between the convenience of disposables, the innovation of smart and portable devices, the expansion into home care and emerging markets, and an increasing focus on efficiency, patient experience, and regulatory compliance. Success will depend on manufacturers’ ability to innovate, address diverse user needs, and navigate evolving healthcare delivery models.

Common Pitfalls Sourcing Nebulisation Kits (Quality, IP)
When sourcing nebulisation kits—especially for medical or industrial applications—organizations often encounter critical challenges related to product quality and intellectual property (IP) risks. Overlooking these aspects can lead to regulatory non-compliance, patient safety issues, legal liabilities, and reputational damage. Below are key pitfalls to avoid:
Quality-Related Pitfalls
1. Inadequate Material Compliance
Many low-cost nebulisation kits use substandard plastics or elastomers that are not biocompatible or resistant to medications. This can lead to leaching of harmful chemicals, device degradation, or inconsistent aerosol delivery—posing serious health risks.
2. Poor Manufacturing Standards
Suppliers, particularly from unverified sources, may lack ISO 13485 certification or Good Manufacturing Practice (GMP) compliance. This increases the risk of contamination, dimensional inaccuracies, and inconsistent performance, undermining device reliability.
3. Inconsistent Aerosol Performance
A major quality issue is variability in particle size distribution (MMAD) and delivered dose. Poorly designed or manufactured kits fail to meet clinical efficacy standards, reducing treatment effectiveness, especially in respiratory therapies.
4. Lack of Regulatory Documentation
Some suppliers provide incomplete or falsified regulatory paperwork (e.g., CE marking, FDA 510(k) clearance, or technical files). This exposes buyers to non-compliance penalties and market access restrictions.
5. Insufficient Testing and Validation
Reputable kits require rigorous performance testing (e.g., flow rate, particle size, durability). Sourcing without access to validation reports increases the risk of deploying underperforming or unsafe devices.
Intellectual Property (IP)-Related Pitfalls
1. Risk of IP Infringement
Many generic or copycat nebulisation kits replicate patented designs (e.g., specific nozzle configurations, valve mechanisms, or chamber geometries). Sourcing such products—even unknowingly—can expose your organization to infringement lawsuits and product seizures.
2. Counterfeit or Grey Market Products
Unauthorized suppliers may offer “compatible” kits that mimic branded designs. These often violate IP rights and lack quality control, blurring the line between legal compatibility and illegal imitation.
3. Unclear IP Ownership in Custom Designs
When co-developing or customizing kits, failure to define IP ownership in contracts can result in disputes. Suppliers may claim rights over design improvements, limiting your freedom to manufacture or modify.
4. Weak Supplier IP Due Diligence
Procurement teams often neglect to audit a supplier’s IP portfolio or freedom-to-operate (FTO) analysis. This oversight increases exposure to third-party patent claims and supply chain disruptions.
5. Branding and Trademark Violations
Some kits may bear logos or packaging resembling established brands, misleading end users and exposing buyers to trademark infringement claims.
Mitigation Strategies
- Conduct thorough supplier audits, including site visits and quality management system reviews.
- Require full regulatory documentation and independent test reports.
- Perform IP clearance searches before finalizing sourcing decisions.
- Use legally reviewed contracts that specify IP ownership, liability, and compliance obligations.
- Partner with reputable manufacturers with proven regulatory and IP compliance histories.
Avoiding these pitfalls ensures not only product safety and efficacy but also protects your organization from legal and operational risks.
H2: Logistics & Compliance Guide for Nebulisation Kit
This guide outlines the essential logistics and compliance considerations for the safe, legal, and efficient handling, storage, transportation, and use of Nebulisation Kits. Adherence to these guidelines is critical to ensure patient safety, product efficacy, and regulatory compliance.
H3: Regulatory Compliance
- Classification & Registration:
- Confirm the regulatory classification of the Nebulisation Kit (e.g., Medical Device Class I, IIa, IIb – specific class depends on design and intended use) in the target markets (e.g., FDA 510(k) clearance/approval in the US, CE Marking under MDR/IVDR in the EU, TGA listing in Australia, Health Canada license).
- Ensure the kit and all components are registered with the relevant national regulatory authorities (e.g., FDA Establishment Registration, EU Authorized Representative).
- Labeling & Instructions for Use (IFU):
- Labels must comply with regional regulations (e.g., FDA 21 CFR Part 801, EU MDR Annex I, ISO 15223-1). Include:
- Product name, UDI (Unique Device Identifier), lot/batch number, expiration date (if applicable).
- Manufacturer name and address, Authorized Representative (EU).
- Intended Use/Indications for Use.
- Contraindications, Warnings, Precautions.
- Storage conditions.
- Single-Use/Multi-Use designation.
- Language requirements (local language(s) in the target market).
- IFUs must be comprehensive, clear, and provided in the required language(s). Include detailed assembly, use, cleaning (if reusable), disinfection/sterilization (if applicable), and disposal instructions.
- Labels must comply with regional regulations (e.g., FDA 21 CFR Part 801, EU MDR Annex I, ISO 15223-1). Include:
- Quality Management System (QMS):
- Manufacturer must operate under a certified QMS (e.g., ISO 13485:2016) covering design, manufacturing, testing, and distribution.
- Maintain records for design history, device master record (DMR), device history record (DHR), and quality audits.
- Post-Market Surveillance (PMS) & Vigilance:
- Implement a system to collect, investigate, and report adverse events and field safety corrective actions (FSCAs) to relevant authorities within mandated timelines (e.g., FDA MedWatch, EUDAMED, national competent authorities).
- Conduct periodic safety update reports (PSURs) as required.
H3: Logistics & Distribution
- Storage Conditions:
- Temperature: Store kits according to manufacturer specifications. Typically, store at controlled room temperature (e.g., 15°C – 25°C / 59°F – 77°F). Avoid freezing and excessive heat (>30°C / 86°F) unless specified otherwise. Monitor and document temperature if required.
- Humidity: Store in a dry environment. Avoid high humidity to prevent degradation of packaging or components (especially electronics in some nebulizers).
- Light: Protect from direct sunlight and strong artificial light. Store in original packaging.
- Environment: Store in a clean, well-ventilated area, protected from dust, dirt, and pests. Keep away from strong odors, solvents, and corrosive substances.
- Segregation: Store medical devices separately from non-medical goods, hazardous materials, and food. Segregate expired or quarantined stock.
- Transportation:
- Mode: Suitable for road, air, and sea freight. Ensure compliance with IATA (air) and IMDG (sea) regulations if applicable (rare for kits without hazardous components).
- Packaging: Ship in original, undamaged manufacturer packaging designed to protect against shock, vibration, compression, moisture, and temperature extremes during transit. Use appropriate secondary packaging (e.g., sturdy outer cartons with cushioning).
- Temperature Control: For temperature-sensitive components (e.g., certain medications if bundled, or sensitive electronics), use validated cold chain or ambient-controlled shipping solutions with temperature monitoring devices (e.g., data loggers). Provide temperature excursions documentation if required.
- Handling: Handle packages with care. Follow “Fragile” and “This Way Up” markings. Avoid dropping, stacking excessively, or exposing to water.
- Documentation: Ensure proper shipping documentation (commercial invoice, packing list, air waybill/bill of lading) includes accurate product descriptions, quantities, and UDI information. Include any required certificates (e.g., Certificate of Conformity, Free Sale Certificate).
- Inventory Management:
- Implement FIFO (First-In, First-Out) or FEFO (First-Expired, First-Out) stock rotation.
- Track inventory levels, lot numbers, and expiration dates using a reliable system (e.g., barcode/RFID scanning).
- Conduct regular stocktakes and reconcile inventory records.
- Quarantine and investigate any damaged, expired, or suspect stock. Segregate and dispose of according to procedures.
H3: Handling & Use (User/Healthcare Facility Level)
- Receiving & Inspection:
- Inspect shipments immediately upon arrival for damage to outer packaging.
- Check for correct items, quantities, and lot numbers against the delivery note.
- Verify storage conditions were maintained (e.g., check temperature logger data if used).
- Report any discrepancies or damage immediately to the supplier.
- Storage (Facility Level):
- Store in a designated, secure, clean, and climate-controlled area meeting the manufacturer’s specifications (same as Distribution Storage).
- Maintain clear labeling and organization.
- Ensure easy access while preventing contamination.
- Dispensing/Issuing:
- Verify patient prescription/order and correct kit type.
- Check lot number and expiration date before dispensing.
- Provide patient with the complete kit and IFU in the required language.
- Provide appropriate training on use, cleaning (if reusable), and maintenance.
- Use & Maintenance:
- Follow the IFU precisely for assembly, medication preparation, operation, and cleaning/disinfection.
- Clean reusable components (mask/mouthpiece, tubing, nebulizer cup) after each use according to IFU (typically washing with warm soapy water, rinsing, and air-drying). Disinfect periodically as instructed.
- Replace consumable parts (filters, tubing, cups, masks) according to the manufacturer’s schedule or when damaged/dirty.
- Do not share kits between patients unless specifically designed and validated for multi-patient use with appropriate reprocessing.
- Disposal:
- Dispose of single-use components and expired kits according to local biohazard and medical waste regulations.
- Dispose of electronic nebulizer units (compressor) according to local electronic waste (WEEE) regulations. Do not dispose of in regular household waste.
- Follow IFU disposal instructions.
H3: Key Compliance Documents
- Certificate of Conformity (CE Marking)
- FDA 510(k) Clearance Letter / Approval Letter
- TGA Certificate of Registration / ARTG Entry
- Health Canada Medical Device License
- ISO 13485:2016 Certificate
- Declaration of Conformity (DoC)
- Free Sale Certificate (if exporting)
- Product Labeling and IFU (in required languages)
- UDI Carrier (on label/packaging)
- Post-Market Surveillance Plan & PSURs
- Validated Cleaning/Disinfection Procedures (if applicable)
- Training Records (for users/staff)
Disclaimer: This guide provides general principles. Specific requirements vary significantly by country, region, kit design, and intended use. Always consult the manufacturer’s specific labeling, IFU, and regulatory documentation, and adhere strictly to all applicable local, national, and international laws and regulations. Regulatory authorities and notified bodies provide definitive guidance.
Conclusion for Sourcing Nebulisation Kit:
In conclusion, sourcing nebulisation kits requires a strategic approach that balances quality, cost, regulatory compliance, and reliability of supply. After evaluating potential suppliers, assessing product specifications, and considering factors such as sterility, compatibility with nebulizer devices, and packaging standards, it is evident that selecting an approved and reputable manufacturer is crucial to ensuring patient safety and treatment efficacy.
Prioritizing suppliers with relevant certifications (such as ISO 13485 and CE or FDA approval), a proven track record in medical device production, and the capacity for consistent delivery will support uninterrupted healthcare services. Additionally, establishing long-term partnerships with suppliers who offer scalability and responsive customer support can enhance supply chain resilience.
Ultimately, the successful sourcing of nebulisation kits not only fulfills clinical requirements but also contributes to improved patient outcomes and operational efficiency within healthcare facilities. Regular performance reviews and market assessments should be conducted to ensure continued alignment with evolving quality and demand standards.