The global Doppler ultrasound market is experiencing robust growth, driven by rising demand for non-invasive diagnostic tools and increasing prevalence of cardiovascular diseases. According to Grand View Research, the global ultrasound imaging market was valued at USD 7.9 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 5.8% from 2023 to 2030. M-mode Doppler ultrasound, a critical modality for assessing cardiac function through precise motion analysis of heart structures, represents a key segment within this expanding market. Advancements in portability, image resolution, and AI integration are accelerating adoption across hospitals, diagnostic centers, and outpatient clinics. As healthcare systems prioritize early diagnosis and point-of-care imaging, leading manufacturers are innovating to meet clinical demands. This momentum has intensified competition among key players, setting the stage for rapid technological evolution. Below, we outline the top 9 M-mode Doppler ultrasound manufacturers shaping the industry’s future through innovation, regulatory milestones, and global reach.
Top 9 M Mode Doppler Ultrasound Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Ultrasound
Domain Est. 2002
Website: butterflynetwork.com
Key Highlights: Butterfly’s Ultrasound-on-chip technology delivers sharp image quality and exciting capabilities, all in a single, whole-body handheld probe….
#2 Spectral doppler ultrasound system
Website: medicalexpo.com
Key Highlights: Find your spectral doppler ultrasound system easily amongst the 199 products from the leading brands (esaote, SonoScape, CONTEC, ….
#3 Musculoskeletal
Domain Est. 1987
Website: usa.philips.com
Key Highlights: Lumify handheld ultrasound for musculoskeletal can help detect soft tissue injuries with high-definition imaging of the muscles, joints, ligaments, ……
#4 Rimed Ltd
Domain Est. 1998
Website: rimed.com
Key Highlights: The company now offers a new line of digital transcranial Dopplers with M-Mode as well as an integrated carotid imaging probe. READ MORE….
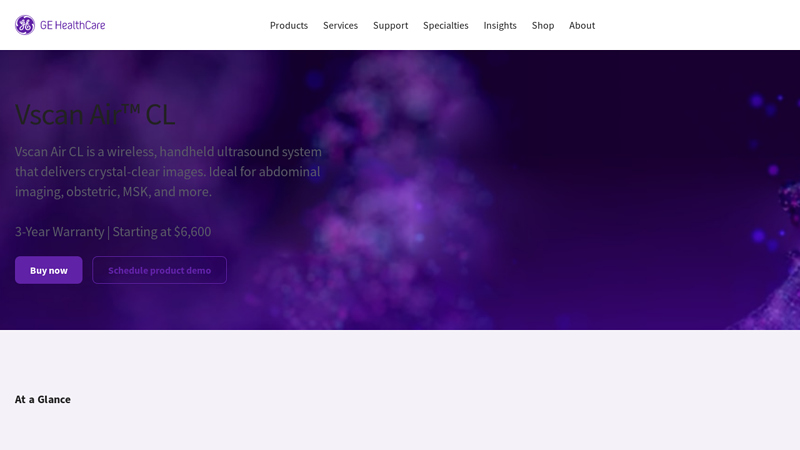
#5 Vscan Air™ CL
Domain Est. 2004
Website: gehealthcare.ca
Key Highlights: Vscan Air CL is a wireless, handheld ultrasound system that delivers crystal-clear images. Ideal for abdominal imaging, obstetric, MSK, and more….
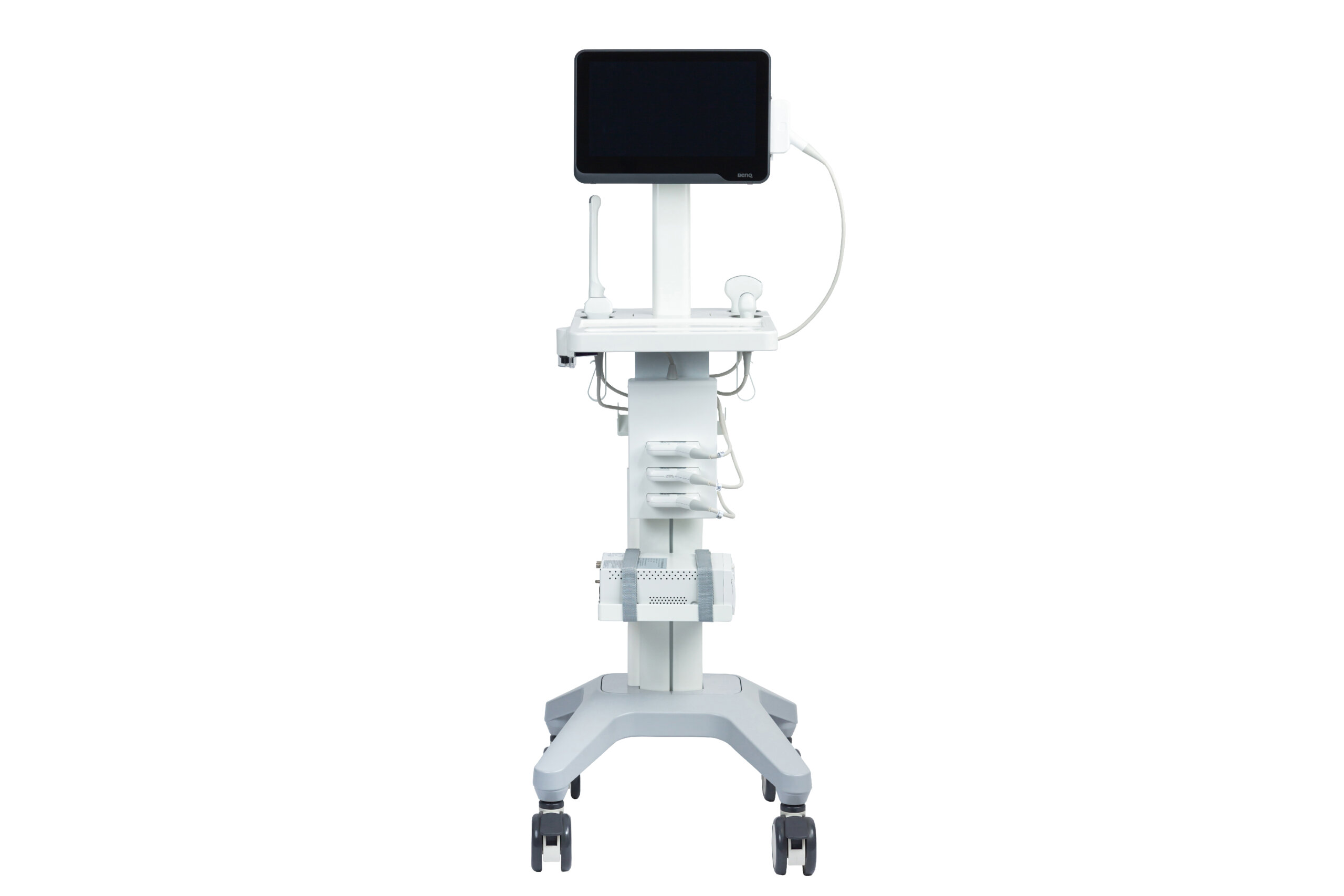
#6 T3300 T
Domain Est. 2011
Website: benqmedicaltech.com
Key Highlights: Portable ultrasound system designed for point-of-care (POC) with high-quality image and lightweight models design which allow you to provide professional and ……
#7 Vave Health: Vave Universal Probe
Domain Est. 2015
Website: vavehealth.com
Key Highlights: Imaging Modes: B-mode, Deep mode, M-mode, & Color Doppler. New presets just dropped MSK, Soft Tissue & More. Always Updated: Benefit from continuous app ……
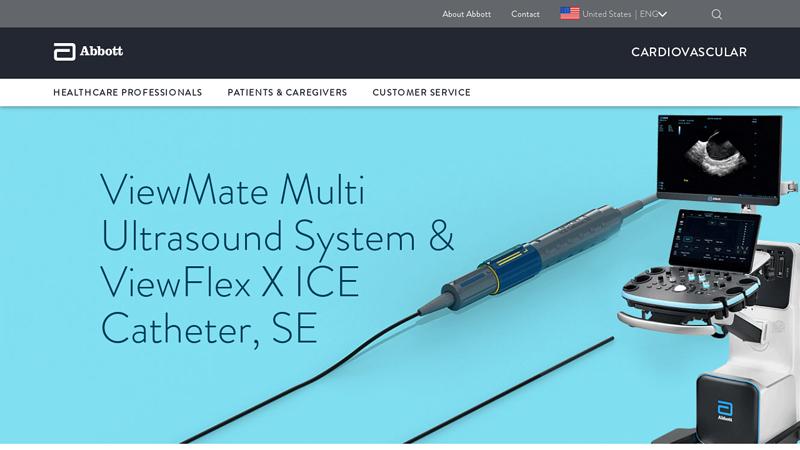
#8 ViewMate Multi Ultrasound System & ViewFlex X ICE Catheter, SE
Domain Est. 2018
Website: cardiovascular.abbott
Key Highlights: ViewMate™ Ultrasound Console with ViewFlex™ Xtra ICE Catheter provides clear visualization of intracardiac anatomy and accurate interpretation of blood flow ……
#9 Medical Ultrasound
Domain Est. 2018
Website: truthbiomedical.com
Key Highlights: TRUTH BIOMEDICAL has the Vast Range of Medical Ultrasound for Healthcare Organization , We also have lots of Advanced Features in Our Medical Ultrasound Range….
Expert Sourcing Insights for M Mode Doppler Ultrasound
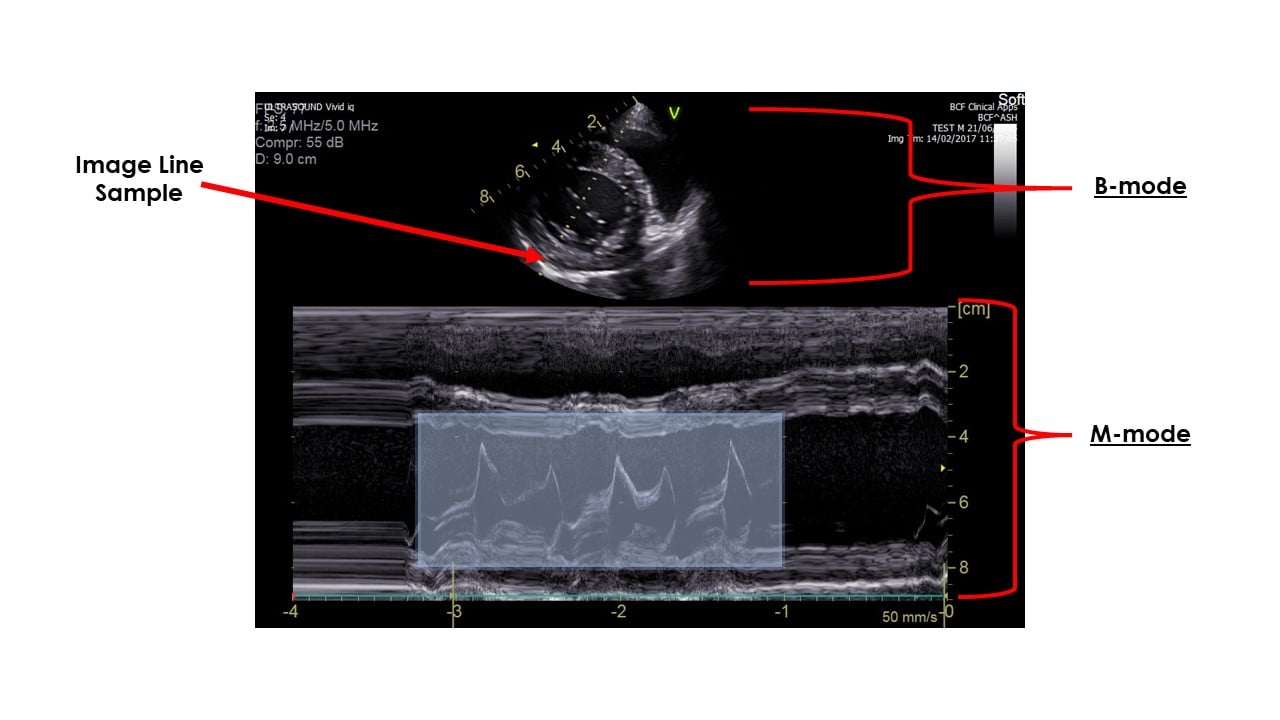
2026 Market Trends for M-Mode Doppler Ultrasound
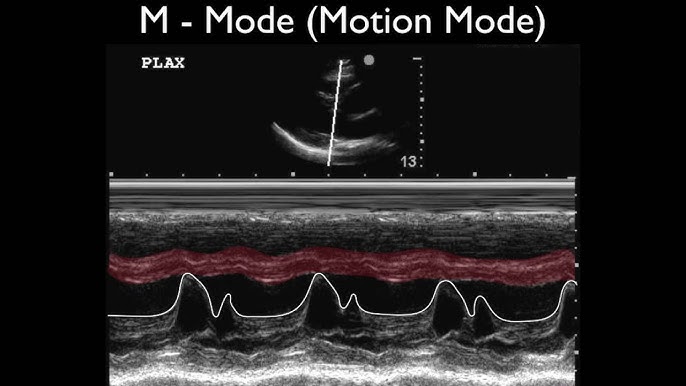
M-Mode (Motion Mode) Doppler Ultrasound, a foundational imaging technique in echocardiography, continues to evolve within the broader ultrasound market landscape. While facing competition from advanced modalities, specific trends are shaping its trajectory heading into 2026.
Integration with Advanced Echocardiography Drives Clinical Utility
By 2026, the primary growth driver for M-Mode Doppler is its seamless integration within comprehensive echocardiography systems. M-Mode’s unparalleled temporal resolution remains critical for precise assessment of cardiac chamber dimensions, wall thickness, and valve motion timing (e.g., mitral valve E-point septal separation for LV function, aortic valve opening/closing). Its synergy with 2D imaging (for precise beam placement) and Doppler modalities (Pulsed-Wave, Continuous-Wave, Color Flow) within modern ultrasound platforms ensures its continued indispensability in standard protocols for diagnosing cardiomyopathies, valvular heart disease, and pericardial effusion. Demand will be sustained by clinical necessity in both routine screenings and complex cardiac assessments.
Cost-Effectiveness and Portability Fuel Adoption in Emerging Markets & Point-of-Care
M-Mode’s technical simplicity and lower computational demands compared to 3D or strain imaging make it highly suitable for integration into cost-effective and portable ultrasound devices. This trend is accelerating its adoption in resource-limited settings (Rural Clinics, Community Health Centers) and for point-of-care (POC) applications (Emergency Departments, Critical Care, Pre-hospital settings). Handheld and compact ultrasound systems featuring robust M-Mode capabilities are becoming essential tools for rapid cardiac assessment, triage, and monitoring, particularly in regions where access to advanced cardiology services is limited. This expanding POC market represents a significant growth vector.
AI and Automation Enhance Efficiency and Standardization
Artificial Intelligence (AI) and machine learning are poised to significantly impact M-Mode usage by 2026. AI algorithms are being developed to automate the placement of the M-Mode cursor on anatomical landmarks (e.g., mitral valve leaflets, interventricular septum) from 2D images, drastically reducing operator dependency and acquisition time. Furthermore, AI-driven analysis can automatically measure standard parameters (LV dimensions, ejection fraction via Teichholz or area-length methods, fractional shortening) from M-Mode tracings, improving measurement accuracy, reproducibility, and workflow efficiency. This automation addresses key challenges of variability and time consumption.
Competition from Advanced Modalities Necessitates Strategic Positioning
The M-Mode Doppler market faces persistent pressure from more advanced quantitative techniques. Speckle Tracking Echocardiography (Strain Imaging) offers superior sensitivity for detecting subtle myocardial dysfunction. 3D Echocardiography provides more accurate volumetric assessments without geometric assumptions. While M-Mode remains a gold standard for specific linear measurements, its role is increasingly complementary. Manufacturers are focusing on bundling M-Mode as a core, reliable component within comprehensive software packages featuring these advanced tools, emphasizing its foundational role in the diagnostic workflow rather than positioning it as a standalone premium feature.
Regulatory and Reimbursement Landscape Influences Access
Reimbursement policies for echocardiography procedures, which often bundle M-Mode assessment, will continue to influence adoption rates. Stable or favorable reimbursement for standard echo studies supports sustained use. Regulatory approvals for AI-powered M-Mode analysis tools will be crucial for their clinical uptake. Furthermore, initiatives promoting standardized echo protocols (e.g., ASE/EACVI guidelines) that mandate M-Mode measurements ensure its ongoing clinical relevance and guide market demand.
In conclusion, the M-Mode Doppler Ultrasound market in 2026 will be characterized by resilience through integration, growth driven by portability and POC applications, enhancement via AI automation, and strategic adaptation amidst competition. Its fundamental value in cardiac assessment ensures its enduring presence, albeit increasingly as an automated, integrated component within sophisticated ultrasound ecosystems rather than a standalone technology.
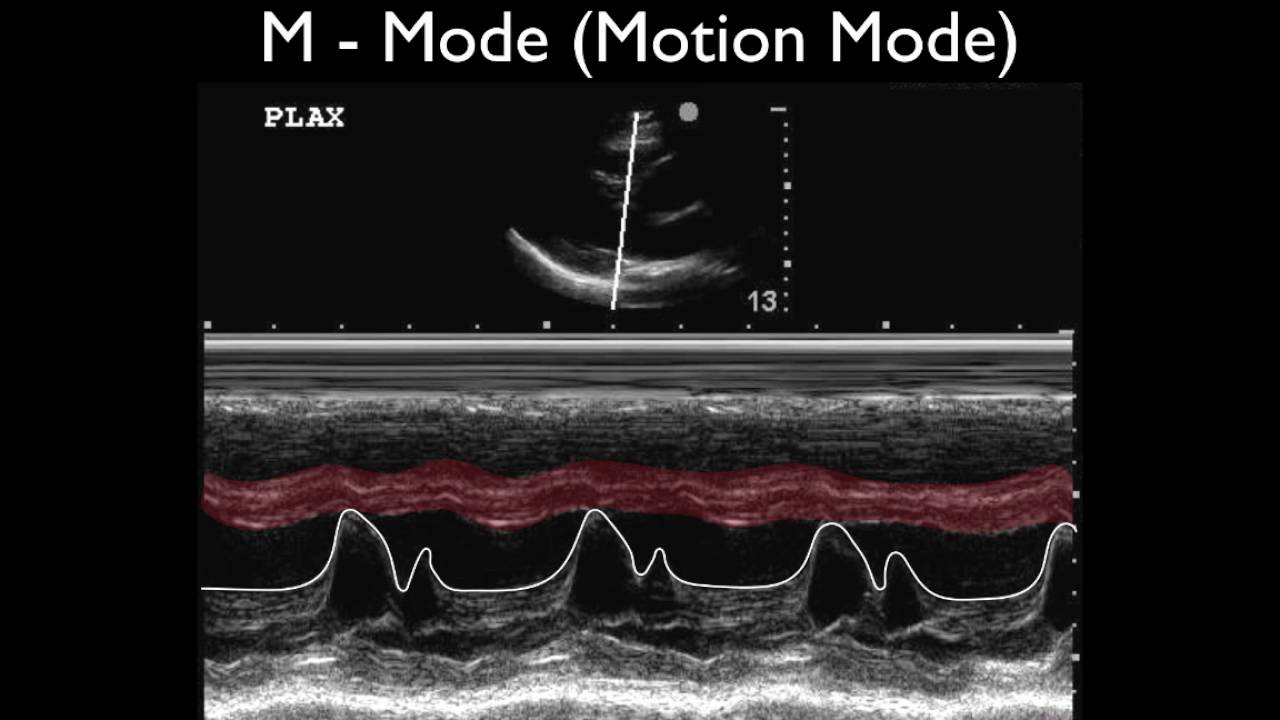
Common Pitfalls in Sourcing M-Mode Doppler Ultrasound: Quality and Intellectual Property Concerns
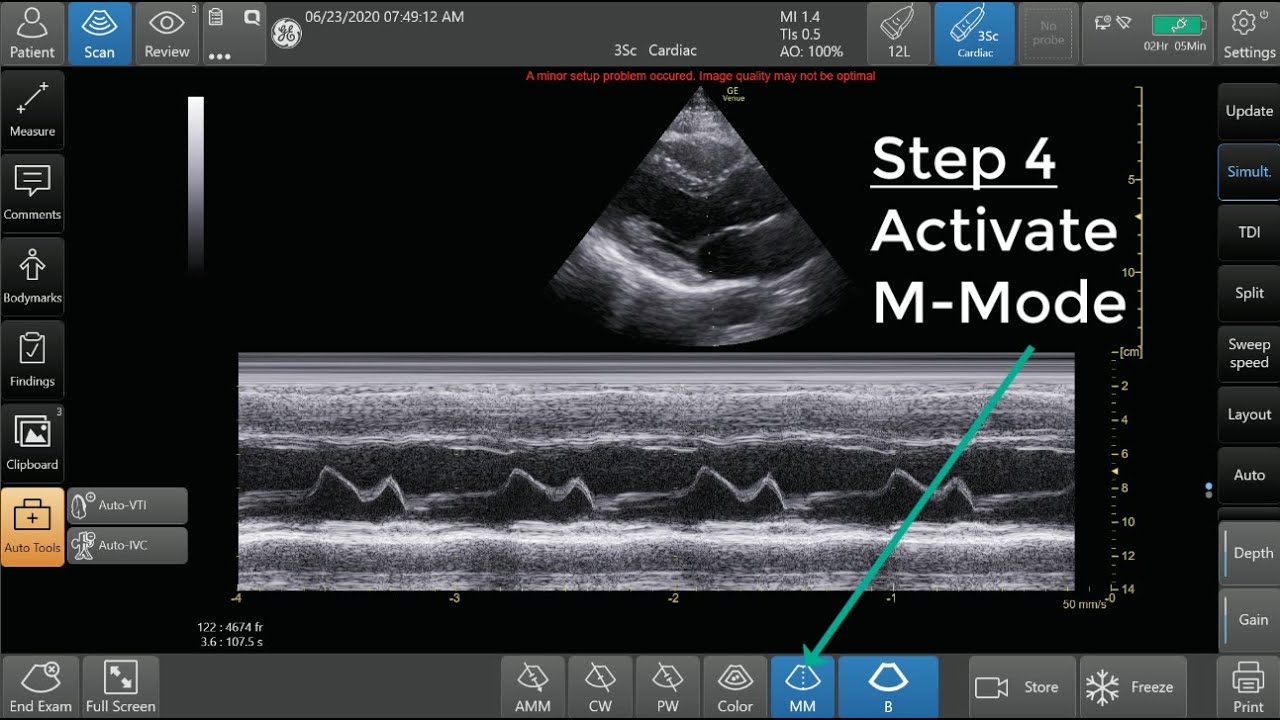
Logistics & Compliance Guide for M-Mode Doppler Ultrasound
This guide outlines the essential logistical considerations and compliance requirements for the safe and effective use of M-Mode Doppler ultrasound in clinical practice.
Equipment Acquisition and Maintenance
Procure M-Mode Doppler ultrasound systems from reputable manufacturers adhering to international safety standards (e.g., IEC 60601-2-37). Establish a regular maintenance schedule, including routine performance testing and calibration by qualified biomedical engineering personnel. Maintain detailed service records and ensure transducers are inspected for damage prior to each use to prevent patient injury and ensure image quality.
Facility and Workflow Planning
Designate a dedicated ultrasound examination area with adequate space for patient movement, equipment maneuverability, and infection control procedures. Ensure reliable electrical outlets and consider power backup solutions. Optimize workflow by scheduling appropriate time per examination, minimizing patient wait times, and integrating ultrasound reporting into the electronic health record (EHR) system for seamless data transfer and accessibility.
Personnel Training and Credentialing
Only qualified healthcare professionals (e.g., sonographers, physicians with appropriate training) should operate M-Mode Doppler ultrasound equipment. Personnel must complete formal training programs covering ultrasound physics, instrumentation, anatomy, scanning techniques, and Doppler principles specific to M-Mode applications. Maintain up-to-date certifications (e.g., ARDMS, CCI) and participate in ongoing continuing medical education (CME) to ensure competency and adherence to evolving best practices.
Patient Preparation and Scheduling
Provide clear instructions to patients regarding preparation (e.g., fasting for abdominal studies, bladder filling for pelvic exams). Confirm patient identity and clinical indication prior to the examination. Schedule appointments to allow sufficient time for thorough evaluation, including patient history review, scanning, image interpretation, and report generation.
Infection Control and Safety Protocols
Adhere strictly to infection prevention guidelines. Clean and disinfect transducers and equipment surfaces between patients using manufacturer-recommended agents and methods compatible with the device. Utilize probe covers when appropriate. Ensure patient safety by confirming no contraindications exist (e.g., open wounds at scan site) and minimizing scan duration to reduce acoustic exposure, following the ALARA (As Low As Reasonably Achievable) principle.
Regulatory and Accreditation Compliance
Ensure compliance with local, national, and international regulations governing medical devices and radiation-emitting products (e.g., FDA, EMA). Participate in accreditation programs (e.g., IAC, ACR) which establish quality benchmarks for ultrasound practices. Maintain accurate documentation of equipment logs, quality assurance data, and patient records to meet audit requirements and support legal defensibility.
Data Management and Privacy
Securely store ultrasound images and reports in compliance with data protection regulations (e.g., HIPAA, GDPR). Implement access controls to ensure only authorized personnel can view or modify patient data. Utilize encrypted storage and transmission methods when sharing studies electronically. Retain records for the legally mandated period as defined by institutional and jurisdictional policies.
Quality Assurance and Continuous Improvement
Implement a comprehensive quality assurance (QA) program including regular review of image quality, measurement accuracy, and report turnaround times. Conduct peer review sessions and participate in external QA programs. Collect and analyze performance metrics to identify areas for improvement and ensure consistent delivery of high-standard care.
Conclusion on Sourcing M-Mode Doppler Ultrasound Equipment
Sourcing M-mode Doppler ultrasound equipment requires a strategic approach that balances clinical needs, budget constraints, and long-term reliability. M-mode ultrasound, which provides high temporal resolution imaging of moving structures—particularly in cardiac and fetal assessments—remains a vital diagnostic tool despite advances in newer modalities. When sourcing this equipment, it is essential to consider factors such as image quality, device portability, integration with existing systems, regulatory compliance (e.g., FDA, CE marking), and availability of technical support and training.
Purchasing new equipment offers the advantage of warranty, updated technology, and better compatibility with current software platforms, while refurbished or pre-owned systems can provide significant cost savings if sourced from reputable vendors with stringent quality control. Additionally, evaluating suppliers based on service agreements, response times, and user feedback ensures sustained operational efficiency.
In conclusion, successful sourcing of M-mode Doppler ultrasound systems depends on a clear understanding of clinical requirements, thorough vendor assessment, and a focus on total cost of ownership. By aligning procurement decisions with quality, reliability, and support, healthcare providers can ensure optimal patient care and maximize the return on their imaging investment.