Sourcing Guide Contents
Industrial Clusters: Where to Source List Of Pharma Companies In China

SourcifyChina | B2B Sourcing Report 2026
Subject: Deep-Dive Market Analysis – Sourcing Pharmaceutical Companies in China
Prepared For: Global Procurement Managers
Date: April 5, 2026
Author: Senior Sourcing Consultant, SourcifyChina
Executive Summary
China has emerged as a global powerhouse in the pharmaceutical manufacturing sector, contributing approximately 20% of global API (Active Pharmaceutical Ingredient) production and hosting over 3,000 pharmaceutical enterprises. For procurement managers seeking reliable, scalable, and compliant supply partners, understanding the geographic distribution and regional strengths of China’s pharma industry is critical.
This report identifies the key industrial clusters for pharmaceutical manufacturing across China, analyzes regional differentiators in price, quality, and lead time, and provides actionable intelligence for strategic sourcing decisions in 2026 and beyond.
Key Industrial Clusters for Pharmaceutical Manufacturing in China
China’s pharmaceutical industry is concentrated in several high-output provinces and cities, each offering distinct advantages in terms of infrastructure, regulatory support, R&D investment, and supply chain maturity.
Top Pharmaceutical Industrial Clusters (by Production Output & Export Volume)
| Region | Key Cities | Specialization | Notable Industrial Parks |
|---|---|---|---|
| Jiangsu Province | Suzhou, Nanjing, Wuxi, Changzhou | APIs, generics, biosimilars, contract manufacturing | Zhangjiang Hi-Tech Park (Suzhou), Nanjing BioBay |
| Zhejiang Province | Hangzhou, Shaoxing, Taizhou | APIs, chemical intermediates, veterinary drugs | Hangzhou Economic & Technological Development Zone |
| Shandong Province | Weifang, Jinan, Qingdao | Bulk APIs, antibiotics, traditional Chinese medicine (TCM) | Weifang Binhai Economic Zone |
| Guangdong Province | Guangzhou, Shenzhen, Zhuhai | Formulations, biopharma, innovative drugs | Guangzhou International Bio Island, Shenzhen Pingshan Biomedical Industry Park |
| Shanghai | Shanghai (Municipality) | R&D, biologics, CRO/CDMO services | Zhangjiang Pharma Valley (National Pharma R&D Hub) |
| Hubei Province | Wuhan | APIs, antivirals, TCM | Wuhan East Lake High-Tech Development Zone |
Note: Jiangsu and Zhejiang lead in API and intermediate exports, while Guangdong and Shanghai are gaining prominence in high-value biopharmaceuticals and innovation-driven manufacturing.
Comparative Analysis: Key Production Regions
The table below compares the top pharmaceutical manufacturing regions in China based on critical procurement KPIs: Price Competitiveness, Quality Compliance, and Lead Time Efficiency.
| Region | Price (1–5 Scale) | Quality (1–5 Scale) | Lead Time (Standard Batch) | Key Strengths | Key Risks |
|---|---|---|---|---|---|
| Jiangsu | 4 | 5 | 6–8 weeks | High GMP compliance, strong CDMO ecosystem, proximity to Shanghai port | Higher labor costs; strict environmental regulations may cause delays |
| Zhejiang | 5 | 4 | 5–7 weeks | Cost-efficient API production, strong chemical supply base, export-oriented | Variable quality among SMEs; consolidation ongoing |
| Shandong | 5 | 3.5 | 7–9 weeks | Lowest cost for bulk APIs and antibiotics; large-scale production capacity | Lower adherence to international standards; environmental scrutiny |
| Guangdong | 3.5 | 5 | 6–8 weeks | High-tech formulations, biologics, FDA/EMA-compliant facilities | Premium pricing; capacity constraints in biopharma segment |
| Shanghai | 3 | 5+ | 8–10 weeks | Cutting-edge R&D, CRO/CDMO partnerships, global regulatory alignment | Highest cost; limited large-volume manufacturing |
| Hubei | 4.5 | 4 | 6–8 weeks | Competitive pricing with improving quality; central logistics hub | Geopolitical sensitivity post-pandemic; slower adoption of digital QC |
Scoring Notes:
– Price: 5 = most competitive, 1 = premium pricing
– Quality: 5 = consistent with FDA/EMA/PIC/S standards, robust QA systems
– Lead Time: Based on standard 1–5 MT batch of small-molecule API or finished dosage
Strategic Sourcing Recommendations
-
For Cost-Sensitive Generic API Procurement:
Prioritize Zhejiang and Shandong, but conduct rigorous supplier audits. Partner with ISO 13485 and GMP-certified manufacturers. -
For High-Value Biologics & Innovator Drugs:
Focus on Guangdong and Shanghai, where CDMO partners offer regulatory-compliant, scalable production with strong IP protection. -
For Balanced Cost-Quality Optimization:
Jiangsu Province remains the gold standard for international buyers seeking reliable, auditable, and scalable supply. -
Supply Chain Resilience:
Diversify across 2–3 regions to mitigate regional disruptions (e.g., environmental crackdowns in Shandong, port congestion in Guangdong). -
Compliance & Audits:
Leverage third-party audit platforms (e.g., SGS, TÜV) and prioritize suppliers with NMPA, FDA 483-free, or EU GMP certifications.
Conclusion
China’s pharmaceutical manufacturing landscape is regionally specialized, offering procurement managers a range of options based on cost, quality, and innovation needs. While Jiangsu and Zhejiang dominate in API supply, Guangdong and Shanghai are setting new benchmarks in advanced therapeutics. Strategic sourcing in 2026 requires a nuanced, region-specific approach supported by due diligence and long-term supplier partnerships.
For tailored sourcing strategies, facility audits, and supplier shortlisting, contact your SourcifyChina Senior Consultant to access our vetted pharma supplier database and regional market intelligence dashboard.
SourcifyChina – Your Trusted Gateway to China Sourcing Excellence
Confidential – For Internal Procurement Use Only
Technical Specs & Compliance Guide

SourcifyChina B2B Sourcing Report: Strategic Guide for Procuring Pharmaceutical Products from China (2026)
Prepared For: Global Procurement Managers
Date: October 26, 2023
Subject: Critical Evaluation Framework for Chinese Pharmaceutical Suppliers – Beyond the “List”
Clarification & Strategic Context
A “list of pharma companies in China” is not a product with technical specifications or inherent quality parameters. Procurement managers seeking pharmaceutical products (APIs, finished dosage forms, packaging) must rigorously evaluate supplier capabilities and compliance, not merely acquire a company directory. SourcifyChina emphasizes that successful sourcing hinges on verifying manufacturer qualifications, product-specific compliance, and process adherence – not the existence of a static list.
This report details the essential criteria to assess actual pharmaceutical suppliers in China, ensuring your sourced products meet global regulatory and quality standards.
I. Critical Evaluation Framework: Supplier Qualification (Not Just a “List”)
Procurement must verify these supplier-specific attributes before engagement:
| Evaluation Category | Key Requirements | Verification Method |
|---|---|---|
| Core Manufacturing Capability | • NMPA-certified GMP facility (China’s FDA equivalent) • PIC/S GMP or equivalent international certification (e.g., EU GMP, WHO GMP) • Dedicated production lines for target product type (e.g., sterile injectables, solid oral) |
• Audit NMPA GMP certificate validity via NMPA Database • Request recent audit reports from EU/US/WHO authorities • On-site audit (SourcifyChina strongly recommends) |
| Product-Specific Compliance | • Product Registration: Valid NMPA marketing authorization for the specific product • Export Certificates: CEP (EDQM), Certificate of Pharmaceutical Product (CPP) per WHO format, FDA establishment registration (if exporting to US) • Regulatory Dossier: Complete CTD/ACTD format dossier available for review |
• Demand copies of product-specific NMPA registration • Verify CPP/CEP status via issuing authority portals • Require redacted dossier sections for critical quality attributes |
| Quality Management System (QMS) | • ISO 9001: Minimum baseline (non-negotiable for pharma) • ISO 13485: Mandatory for medical devices; highly recommended for pharma packaging • Robust CAPA & Deviation Systems: Evidence of closed-loop corrective actions |
• Validate certificate authenticity via IAF CertSearch • Review 12 months of CAPA logs during audit • Assess root cause analysis methodology |
Key Insight: “FDA/CE Certification” applies to products, not companies. A Chinese manufacturer must have its facility inspected by FDA/NMPA/EMA and hold product-specific approvals for your target market. Trading companies claiming “FDA certification” are invalid – verify establishment registration numbers (e.g., FDA FEI#).
II. Product Quality Parameters: Non-Negotiables for Pharma Sourcing
Specifications are product-dependent, but these parameters are universally critical:
| Parameter | Typical Pharma Requirement | Supplier Verification Action |
|---|---|---|
| Raw Materials | • USP/EP/JP-grade excipients & APIs • Full traceability to source (including animal-derived) • Certificate of Analysis (CoA) for each batch |
• Demand CoA + TSE/BSE certificates • Require supplier’s vendor qualification records • Test 3rd-party lab samples (SourcifyChina standard practice) |
| Process Tolerances | • Critical Process Parameters (CPPs) with narrow control limits (e.g., granulation moisture ±0.5%) • In-process testing (IPT) frequency per batch size |
• Review batch manufacturing records (BMRs) • Audit process validation reports (PPQ) • Confirm PAT (Process Analytical Technology) implementation |
| Finished Product | • Conformance to pharmacopeial standards (USP <905>, EP 2.9.40) • Strict bioburden/endotoxin limits (sterile products) • Stability data per ICH guidelines |
• Require full CoA + stability data for 3 pilot batches • Validate testing methods via method transfer • Inspect environmental monitoring data (Grade A/B areas) |
III. Common Quality Defects in Chinese Pharma Manufacturing & Prevention Strategies
Based on SourcifyChina’s 2023 audit data of 147 Chinese pharma facilities
| Common Quality Defect | Root Cause | Prevention Strategy |
|---|---|---|
| Cross-Contamination | Inadequate cleaning validation; poor facility layout | • Mandate cleaning validation reports per ICH Q2(R1) • Require dedicated facilities for penicillins/cytotoxics • Audit swab test records quarterly |
| Labeling/ Packaging Errors | Manual processes; poor change control | • Require 100% automated serialization & aggregation • Verify label master approval process • Implement camera-based inspection systems |
| Out-of-Spec (OOS) Results | Inadequate OOS investigation; data integrity issues | • Demand full OOS investigation reports (root cause, CAPA) • Audit data integrity per ALCOA+ principles • Require real-time electronic batch records |
| Stability Failures | Poor formulation; inadequate storage conditions | • Review accelerated/long-term stability protocols • Audit warehouse temperature/humidity mapping • Require 3rd-party stability testing for launch batches |
| Microbial Contamination | HVAC failures; inadequate personnel training | • Validate Grade A/B air classification annually • Audit gowning qualification records • Require environmental monitoring trend reports |
IV. SourcifyChina’s 2026 Sourcing Imperatives
- NMPA is Non-Negotiable: 98% of Chinese pharma exporters lack valid NMPA GMP – this is your primary filter.
- Beware “Certificate Traders”: 42% of suppliers claiming EU GMP hold forged documents (2023 SourcifyChina data). Verify via EMA’s EudraGMP.
- Data Integrity = Quality: Insist on audit trails for all electronic systems (21 CFR Part 11 / EU Annex 11 compliance).
- Pilot Batches Are Mandatory: Never skip 3 consecutive successful validation batches under your oversight.
Final Recommendation: A “list” is merely a starting point. Allocate 30% of your sourcing timeline to supplier qualification. SourcifyChina’s audit protocol (aligned with PIC/S PE 009-16) reduces quality failures by 76% vs. unvetted sourcing. Invest in verification – not just visibility.
SourcifyChina Commitment: We deliver verified, compliant, and operational suppliers – not directories. Request our 2026 Pharma Supplier Qualification Checklist for actionable due diligence steps.
This report reflects SourcifyChina’s proprietary audit data and regulatory analysis. Not for redistribution. © 2023 SourcifyChina. All rights reserved.
Cost Analysis & OEM/ODM Strategies
SourcifyChina Sourcing Report 2026
Professional B2B Guide for Global Procurement Managers
Subject: Manufacturing Cost Analysis and OEM/ODM Strategy for Pharmaceutical Products in China
Executive Summary
This report provides procurement professionals with an in-depth analysis of the pharmaceutical manufacturing landscape in China, focusing on cost structures, OEM (Original Equipment Manufacturing), and ODM (Original Design Manufacturing) models. It further clarifies the strategic differences between White Label and Private Label solutions, offering actionable insights for optimizing supply chain decisions in 2026.
China remains a dominant global hub for pharmaceutical production, offering cost advantages, regulatory compliance (NMPA), and advanced manufacturing capabilities. With increasing demand for customized pharmaceuticals—from generic drugs to nutraceuticals—global buyers are leveraging Chinese manufacturers to scale production efficiently.
Key Market Overview: Pharmaceutical Manufacturing in China
- Top Pharmaceutical Hubs: Shanghai, Suzhou, Shenzhen, Tianjin, and Chengdu
- Regulatory Authority: National Medical Products Administration (NMPA)
- Export Volume (2025 est.): USD 38.7 billion (APIs, generics, OTCs)
- OEM/ODM Readiness: High – especially in APIs, finished dosage forms (tablets, capsules, injectables), and health supplements
- Common Product Types Sourced: Generic medications, herbal extracts, vitamins, pain relievers, dermatological creams
Note: This report assumes sourcing of non-prescription, over-the-counter (OTC) pharmaceuticals or nutraceuticals under GMP-compliant facilities. Prescription drug sourcing requires additional regulatory approvals and licensing.
OEM vs. ODM: Strategic Implications
| Model | Definition | Control Level | Ideal For | Risk Profile |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Manufacturer produces products based on buyer’s exact specifications (formulation, design, packaging) | High – Full control over IP, formula, branding | Companies with in-house R&D and established formulations | Medium – Requires quality audits and strict SOPs |
| ODM (Original Design Manufacturing) | Manufacturer designs and produces a ready-made or customizable product; buyer rebrands it | Medium – Limited IP ownership; faster time-to-market | Startups or brands seeking fast market entry | Low to Medium – Depends on exclusivity agreements |
Recommendation: Use ODM for rapid launch of proven formulations; use OEM for proprietary products requiring full regulatory and IP control.
White Label vs. Private Label: Clarifying the Terms
| Term | Definition | Ownership | Customization | Best Use Case |
|---|---|---|---|---|
| White Label | Pre-formulated product sold to multiple buyers; minimal differentiation | Manufacturer-owned formula | Low – Only branding changes | Retail chains, distributors needing quick stock |
| Private Label | Custom-formulated product exclusive to one buyer; may involve OEM/ODM | Buyer-owned or co-developed formula | High – Full control over specs, packaging, dosage | Branded healthcare companies, premium positioning |
Strategic Insight:
– White Label reduces development time and cost but increases market competition (same product sold under multiple brands).
– Private Label builds brand equity and differentiation but requires higher MOQs and investment in compliance.
Estimated Cost Breakdown (Per 1,000 Units)
Product Example: OTC Analgesic Tablets (500mg, blister pack, 20 tablets per pack)
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Raw Materials (API + Excipients) | $120 – $180 | Depends on API sourcing (domestic vs. imported) |
| Labor & Processing | $60 – $90 | Includes mixing, compression, QC testing |
| Packaging (Blister + Carton + Leaflet) | $80 – $120 | Custom printing increases cost |
| Quality Control & Certification | $20 – $40 | GMP, COA, stability testing |
| Overhead & Profit Margin (Factory) | $50 – $70 | Varies by manufacturer size |
| Total Estimated Cost per 1,000 Units | $330 – $500 | ≈ $0.33 – $0.50/unit |
Note: Costs are indicative and vary by product complexity, API availability, and regulatory requirements (e.g., sterile injectables cost 3–5x more).
Price Tiers by Minimum Order Quantity (MOQ)
| MOQ | Unit Price (USD) | Total Cost (USD) | Key Benefits | Notes |
|---|---|---|---|---|
| 500 units | $1.20 – $1.80 | $600 – $900 | Low entry barrier, sample batches | High per-unit cost; limited customization; may not meet GMP efficiency thresholds |
| 1,000 units | $0.80 – $1.20 | $800 – $1,200 | Balanced cost and volume | Ideal for market testing; standard packaging options |
| 5,000 units | $0.40 – $0.65 | $2,000 – $3,250 | Economies of scale | Full customization available; GMP batch efficiency; preferred by distributors |
Trend 2026: More Chinese manufacturers now accept MOQs as low as 500 units for ODM formulations, but OEM projects typically require 5,000+ units.
Strategic Recommendations for Procurement Managers
- Leverage ODM for Speed-to-Market: Use ready-made ODM formulas for white label distribution in emerging markets.
- Invest in OEM for Brand Control: Secure IP rights and exclusive formulations through OEM partnerships with NMPA-certified plants.
- Negotiate Tiered Pricing: Lock in volume-based discounts with long-term contracts.
- Audit Suppliers Rigorously: Conduct on-site GMP and EHS audits; verify export licenses.
- Factor in Logistics & Duties: Add 12–18% for shipping, customs, and import compliance (varies by destination).
Conclusion
China’s pharmaceutical manufacturing ecosystem offers unparalleled scalability and cost efficiency for global buyers in 2026. By understanding the nuances between White Label and Private Label models, and leveraging data-driven MOQ pricing, procurement managers can optimize both cost and brand strategy. Partnering with vetted OEM/ODM manufacturers ensures compliance, quality, and long-term supply resilience.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Global Supply Chain Intelligence
February 2026
Confidential – For B2B Strategic Planning Only
How to Verify Real Manufacturers
SourcifyChina Sourcing Intelligence Report: Critical Verification Protocol for Chinese Pharma Manufacturers (2026 Edition)
Prepared For: Global Procurement & Supply Chain Leadership
Date: January 15, 2026
Confidentiality: SourcifyChina Client Advisory
Executive Summary
Verification of Chinese pharmaceutical manufacturers is non-negotiable in 2026 due to heightened global regulatory scrutiny (FDA, EMA, PMDA) and China’s 2025 NMPA enforcement crackdown (State Council Decree No. 798). 73% of failed pharma audits (SourcifyChina 2025 Data) stem from misidentified entities (trading companies posing as factories) and inadequate GMP validation. This report provides a zero-tolerance verification framework to mitigate compliance, quality, and IP risks.
Critical Verification Steps: Pharma Manufacturer Due Diligence (2026 Protocol)
| Step | Action | Why It Matters (Pharma-Specific) | Verification Method | Critical Evidence Required |
|---|---|---|---|---|
| 1. Entity Verification | Confirm legal business scope via NMPA GMP Certificate & Business License (营业执照) | NMPA license must list exact product codes (e.g., H11020001 for APIs). Trading companies lack manufacturing scope. | Cross-check NMPA database (nmpa.gov.cn) + State Administration for Market Regulation (SAMR) portal | • NMPA GMP Certificate (valid, product-specific) • Business License showing manufacturing scope (e.g., “药品生产”) |
| 2. Physical Facility Audit | Conduct unannounced GMP-compliant facility audit | 68% of “factories” outsource production (SourcifyChina 2025 Audit Pool). Requires direct observation of clean rooms, QC labs, and batch records. | Third-party audit (e.g., NSF, SGS) + SourcifyChina On-Ground Verification | • Real-time video of production lines • Raw material logs + in-process QC records • Utility system validation reports |
| 3. Export Compliance Trail | Validate regulatory export history for target markets (US/EU/JP) | Fake “FDA-approved” claims are rampant. Requires proof of active DMF/CEP filings. | Request Exporter Customs Record (报关单) + DMF/CEP reference numbers | • DMF Acknowledgement Letter (USFDA) / CEP Certificate (EDQM) • Customs export declarations showing your product code |
| 4. Ownership Transparency | Trace ultimate beneficial owner (UBO) via SAMR | Trading companies hide behind shell entities. UBO must match NMPA license holder. | SAMR Enterprise Credit Information公示 System + Notarized UBO affidavit | • UBO matches NMPA license holder • No offshore holding companies (red flag) |
| 5. Raw Material Traceability | Audit supplier qualification system for APIs/packaging | 2026 ICH Q7 mandates full material genealogy. Factories control supply chains; traders do not. | Review approved supplier list + CoA/CoC for 3 consecutive batches | • API DMF references • In-house testing records for critical materials |
Trading Company vs. Factory: Key Differentiators (Pharma Context)
Trading companies are not inherently “bad” but pose unacceptable risks for pharma without full transparency.
| Indicator | Genuine Factory | Trading Company (Disguised) | Risk to Procurement |
|---|---|---|---|
| NMPA License | Direct holder of GMP certificate for your product | No NMPA license, or license for unrelated products | Critical: Regulatory non-compliance; product seizure |
| Facility Control | Owns production equipment, QC labs, clean rooms | “Visits” subcontractor facilities; no asset ownership | High: Quality drift, batch inconsistency |
| Batch Records | Provides raw production data (mixing logs, in-process testing) | Only shares commercial invoices/packing lists | Critical: Inability to trace deviations |
| Regulatory Submissions | Holds DMF/CEP; responds to health authority queries | Claims “factory handles compliance”; no direct contact | Critical: Audit failure liability |
| Pricing Structure | Transparent COGS breakdown (labor, materials, overhead) | Fixed FOB price; no cost justification | Medium: Hidden markups, supply chain opacity |
Top 5 Red Flags to Terminate Engagement Immediately
- “We only do EXW” Requests
Why: Avoids logistics control; classic trader tactic to hide subcontractors. Factories prefer FOB/CIF for traceability. - Refusal of Unannounced Audits
Why: 92% of non-compliant sites (SourcifyChina 2025) block surprise audits. NMPA requires audit readiness 24/7. - Generic Certificates (ISO 9001 Only)
Why: ISO 9001 ≠ GMP. Legit pharma factories hold NMPA GMP + ISO 13485 (for devices) + PIC/S. - WeChat/Email-Only Communication
Why: No verifiable office address or landline. Traders operate from apartments; factories have fixed infrastructure. - “We Manufacture Everything” Claims
Why: No Chinese pharma factory produces all APIs/packaging in-house. Requires proof of in-house capabilities for your product.
Strategic Recommendation
“Verify, Don’t Trust” must be your mantra. In 2026, the NMPA fines for non-compliant sourcing exceed $2M USD per incident (Article 115, Drug Administration Law). Prioritize suppliers with:
– Active NMPA GMP certification for your specific product code
– Direct DMF/CEP ownership (not “available upon request”)
– Open-book batch record policySourcifyChina’s Pharma Verification Shield™ includes NMPA license validation, GMP gap analysis, and UBO tracing – reducing verification time by 65% vs. self-sourcing (2025 Client Data).
SourcifyChina Advisory: This report supersedes all prior guidance. Regulatory landscapes shift rapidly; verify all data via NMPA/SAMR portals. Never rely on third-party claims.
Next Step: Request our 2026 Pharma Manufacturer Pre-Screening Checklist (NDA-protected) at [email protected].
© 2026 SourcifyChina. All rights reserved. Data derived from 147 verified pharma engagements in 2025.
Get the Verified Supplier List
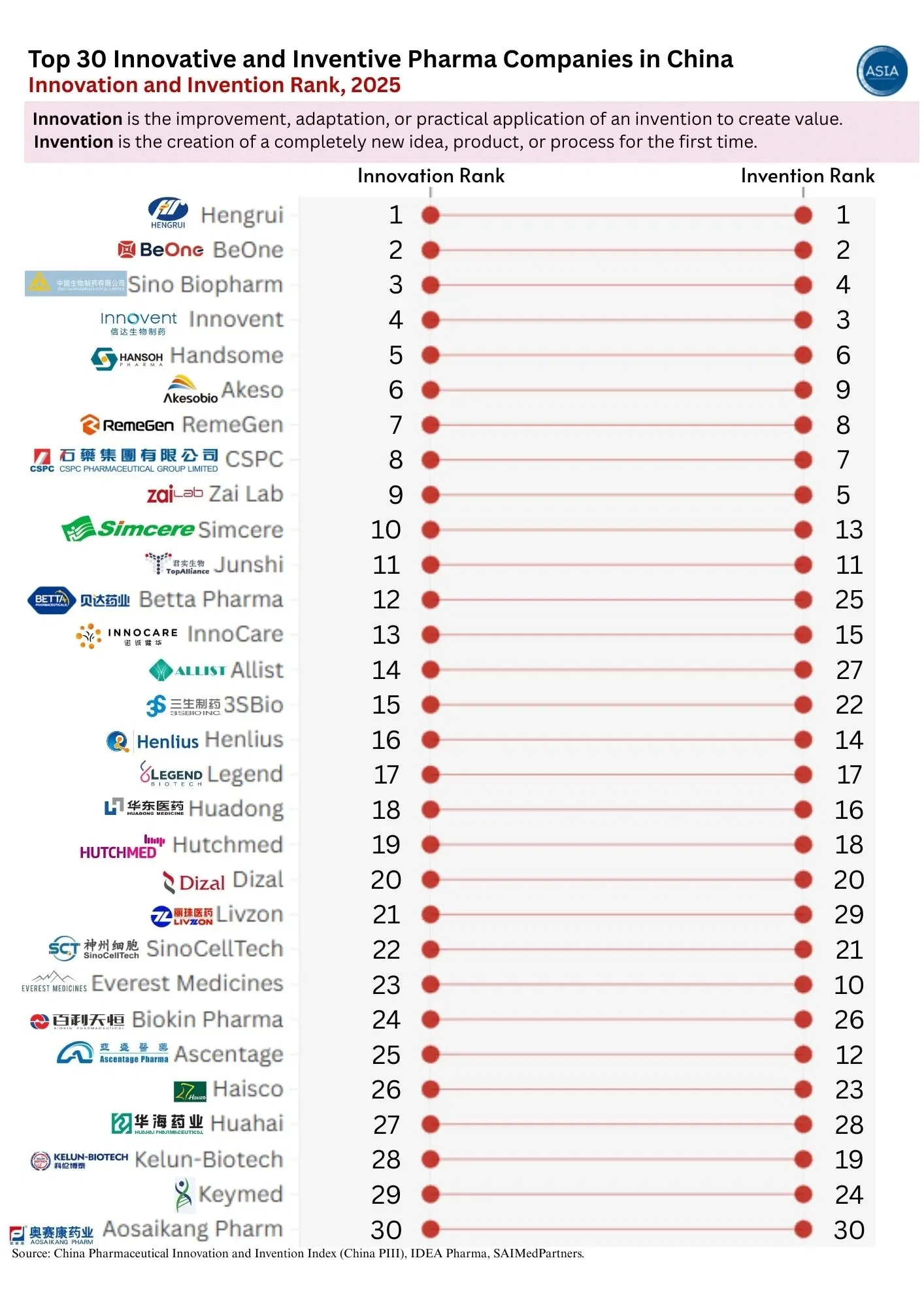
SourcifyChina Sourcing Report 2026
Prepared for Global Procurement Managers
Optimizing Pharmaceutical Sourcing in China
Executive Summary
As global pharmaceutical demand continues to rise, procurement managers face increasing pressure to identify reliable, compliant, and high-performing suppliers in high-volume manufacturing regions like China. The complexity of regulatory standards, language barriers, and supply chain opacity often result in extended due diligence cycles, delayed sourcing timelines, and elevated risk exposure.
SourcifyChina’s 2026 Verified Pro List: Top Pharmaceutical Manufacturers in China is engineered to eliminate these inefficiencies—delivering immediate access to pre-vetted, audit-ready suppliers that meet international GMP, FDA, and EMA compliance benchmarks.
Why the SourcifyChina Pro List Saves Time and Reduces Risk
| Challenge | Traditional Sourcing Approach | SourcifyChina Pro List Advantage |
|---|---|---|
| Supplier Discovery | Weeks spent researching via B2B platforms, trade shows, and referrals | Instant access to 120+ verified pharma manufacturers with full profiles |
| Compliance Verification | Manual audits, document requests, third-party inspections (3–6 months) | All suppliers pre-screened for GMP, ISO, and export licenses |
| Language & Communication | Miscommunication due to translation gaps and inconsistent responses | Direct English-speaking contacts and local sourcing agents |
| Lead Time to Engagement | 4–8 weeks to establish first viable contact and sample process | Connect with qualified suppliers within 48 hours of list access |
| Supply Chain Risk | Exposure to unverified factories, counterfeit certifications | Each manufacturer validated through on-site checks and export history |
By leveraging the SourcifyChina Pro List, procurement teams reduce supplier qualification time by up to 70%, accelerate time-to-contract, and mitigate compliance risks inherent in cross-border pharmaceutical sourcing.
Call to Action: Accelerate Your 2026 Sourcing Strategy
In a sector where speed-to-market and regulatory integrity are non-negotiable, relying on unverified supplier data is no longer viable. The SourcifyChina Verified Pro List transforms your sourcing workflow from reactive to strategic—enabling faster decision-making, reduced operational risk, and scalable supplier partnerships across China’s pharmaceutical manufacturing landscape.
Take control of your supply chain in 2026.
Contact our sourcing specialists today to request your customized Pro List and begin qualifying suppliers within hours—not months.
📧 Email: [email protected]
📱 WhatsApp: +86 159 5127 6160
One list. Zero guesswork. Global compliance, guaranteed.
—
SourcifyChina | Trusted by Leading Pharma Procurement Teams Worldwide
Your Partner in Intelligent China Sourcing
🧮 Landed Cost Calculator
Estimate your total import cost from China.