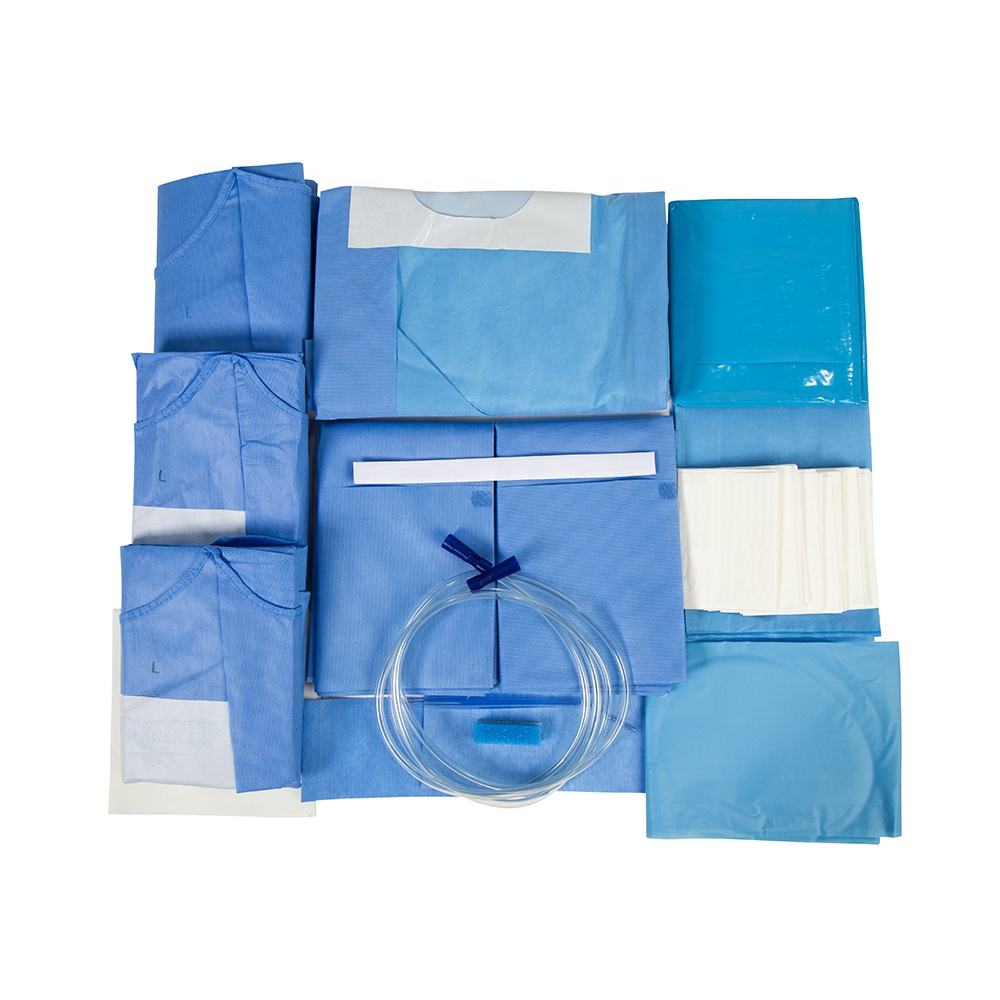
The global surgical drapes market is experiencing steady growth, driven by rising surgical volumes, increasing adoption of specialized surgical procedures, and heightened focus on infection control in healthcare settings. According to Grand View Research, the global surgical drapes market was valued at USD 2.8 billion in 2023 and is projected to expand at a compound annual growth rate (CAGR) of 6.2% from 2024 to 2030. This growth trajectory is further supported by advancements in drape materials, including fluid-resistant barriers and antimicrobial technologies, particularly in high-acuity procedures such as laparotomies. As demand for sterile, procedure-specific drapes rises, manufacturers are focusing on innovation, customization, and compliance with stringent regulatory standards. In this evolving landscape, a select group of companies has emerged as leaders in the laparotomy drape segment, combining clinical efficacy with scalable manufacturing—setting the benchmark for quality and performance in modern operating rooms.
Top 6 Laparotomy Drape Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Laparotomy StanGuard Surgical Drapes
Domain Est. 1995
Website: standardtextile.com
Key Highlights: Laparotomy StanGuard Surgical Drapes are fenestrated drape sheets that are used to isolate the surgical site from other areas of the patient’s body….
#2 Laparotomy drapes and sets
Domain Est. 1996
Website: molnlycke.com
Key Highlights: The surgical patient drapes are intended to be used as protective patient coverings to create a sterile field for a surgical procedure. Show moreShow less ……
#3 Disposable Surgical Drapes for Laparotomy
Domain Est. 1998
Website: winnermedical.com
Key Highlights: A laparotomy drape is a disposable surgical drape designed for minimally invasive and other procedures on the abdomen….
#4 Laparotomy Drape
Domain Est. 2006
Website: welmed.us
Key Highlights: Laparotomy Drape. Overall Dimensions: 106″ x 77″ x 120″; Arm Board Covers; 30″ x 24″ Reinforcement Surrounding Fenestration; 4″ x 12″ Fenestration with ……
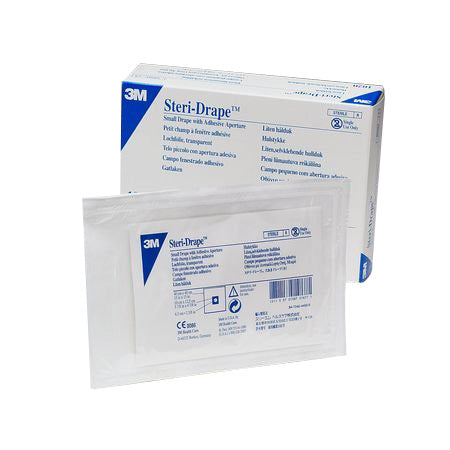
#5 3M™ Steri
Domain Est. 2007
Website: solventum.com
Key Highlights: Product ID. 7000002774. Overall Width (Imperial). 89.76 in. Overall Width (Metric). 228 cm. Overall Length (Imperial). 84.25 in. Overall Length (Metric)….
#6 HALYARD* Laparotomy Drapes
Domain Est. 2013
Website: products.halyardhealth.com
Key Highlights: From laparotomy and endoscopic drapes to breast chest and thyroid drapes HALYARD* has adaptable systems that offer maximum flexibility for surgical procedures….
Expert Sourcing Insights for Laparotomy Drape
H2: Projected Market Trends for Laparotomy Drapes in 2026
The global market for laparotomy drapes is poised for steady growth by 2026, driven by increasing surgical volumes, advancements in infection control protocols, and rising demand for specialized surgical drapes in minimally invasive and open procedures. The following key trends are expected to shape the laparotomy drape market in 2026:
-
Increased Focus on Infection Prevention
With healthcare-associated infections (HAIs) remaining a critical concern, hospitals and surgical centers are prioritizing high-barrier, fluid-resistant drapes. Laparotomy drapes with enhanced antimicrobial coatings, multi-layer designs, and integrated adhesive borders are expected to gain traction. Regulatory bodies and accreditation organizations are likely to reinforce standards around surgical site infection (SSI) reduction, further boosting demand for high-performance drapes. -
Shift Toward Customized and Procedure-Specific Drapes
Surgeons are increasingly opting for procedure-specific surgical packs, including tailored laparotomy drapes with pre-positioned fenestrations, pouches for specimen collection, and built-in armboards or fluid management systems. This trend supports surgical efficiency and reduces contamination risks, making customized kits a key growth segment by 2026. -
Growth in Minimally Invasive and Hybrid Procedures
While laparotomy traditionally refers to open abdominal surgery, the integration of hybrid techniques—combining open and laparoscopic approaches—is rising. This drives demand for hybrid-compatible drapes that support both access types, featuring dual fenestrations and enhanced drape stability. Manufacturers are responding with innovative designs to accommodate evolving surgical workflows. -
Expansion in Emerging Markets
Regions such as Asia-Pacific, Latin America, and the Middle East are witnessing rapid expansion in surgical infrastructure and healthcare access. Rising disposable incomes, government investments in healthcare, and growing awareness of sterile surgical practices are fueling market penetration of advanced laparotomy drapes in these regions. -
Sustainability and Eco-Conscious Product Development
Environmental concerns are influencing product design, with major manufacturers exploring biodegradable materials, reduced packaging, and reusable drape systems where appropriate. While disposable drapes will remain dominant due to sterility requirements, sustainability is emerging as a differentiating factor in procurement decisions. -
Consolidation and Strategic Partnerships Among Suppliers
The competitive landscape is expected to see increased mergers, acquisitions, and partnerships between medical textile companies and surgical supply distributors. These collaborations aim to enhance R&D capabilities, broaden product portfolios, and improve supply chain resilience, particularly in the wake of recent global disruptions. -
Digital Integration and Smart Drape Technologies
Although still in early stages, research is underway into “smart” surgical drapes embedded with sensors to monitor fluid leakage, drape integrity, or patient temperature. While not yet mainstream, these innovations may begin pilot adoption in select tertiary care centers by 2026, setting the stage for future market transformation.
In conclusion, the laparotomy drape market in 2026 will be characterized by innovation in materials and design, heightened emphasis on patient safety, and geographic expansion. Manufacturers that align with clinical needs, regulatory trends, and sustainability goals are likely to capture significant market share in this evolving landscape.

Common Pitfalls Sourcing Laparotomy Drapes (Quality, IP)
Sourcing laparotomy drapes involves critical considerations for both clinical safety and legal compliance. Overlooking key pitfalls related to quality and intellectual property (IP) can lead to patient risk, regulatory setbacks, and financial loss.
Poor Material Quality and Inadequate Barrier Performance
One of the primary risks in sourcing laparotomy drapes is selecting products with substandard materials. Low-quality fabrics or coatings may fail to provide an effective microbial barrier, increasing the risk of surgical site infections (SSIs). Drapes that lack sufficient fluid resistance or tear strength can compromise sterility during procedures. Additionally, inadequate adhesive performance might result in drape lift or movement, exposing non-sterile areas.
Non-Compliance with Regulatory Standards
Sourced drapes must meet stringent regulatory requirements such as FDA 510(k), CE marking under MDR, or other local medical device regulations. Failure to verify compliance can result in import bans, product recalls, or legal liability. Suppliers may claim conformity without valid certifications or up-to-date technical documentation, placing the sourcing organization at risk.
Inconsistent Manufacturing and Sterilization Processes
Variability in manufacturing—such as inconsistent coating thickness, adhesive application, or folding techniques—can affect drape performance. Similarly, lapses in sterilization validation (e.g., inadequate EO or gamma radiation protocols) may compromise sterility assurance levels (SAL). Sourcing from facilities without robust quality management systems (e.g., ISO 13485 certification) increases exposure to these risks.
Intellectual Property Infringement Risks
Using or sourcing drapes that mimic patented designs, adhesive patterns, or composite material structures can lead to IP infringement. Some suppliers, particularly in less-regulated markets, may produce “copy” products that violate existing patents held by major medical device companies. Purchasers may face legal action, shipment seizures, or reputational damage even if unaware of the infringement.
Lack of Transparency in Supply Chain and Raw Materials
Opaque supply chains make it difficult to verify the origin of raw materials, such as nonwoven fabrics or adhesives. This opacity increases the risk of counterfeit components or materials containing allergens or restricted substances (e.g., latex, certain chemicals). Without full traceability, it’s challenging to conduct root cause analysis in case of adverse events.
Insufficient Clinical Validation and User Testing
Some sourced drapes may lack proper clinical evaluation or user feedback data. Designs not optimized for surgical workflow—such as poor fenestration placement, inadequate drape size, or lack of accessory pouches—can hinder surgical efficiency and increase contamination risk. Relying solely on technical specs without clinical input may result in unsuitable products.
Overlooking Patent Landscapes and Freedom-to-Operate Analysis
Before sourcing or rebranding a laparotomy drape, it’s essential to conduct a freedom-to-operate (FTO) search. Assuming a product is generic without verifying active patents in target markets can lead to costly litigation. Key patented features often include fluid collection systems, split-back designs, and antimicrobial coatings.
Avoiding these pitfalls requires due diligence, including supplier audits, verification of certifications, IP screening, and collaboration with clinical stakeholders to ensure both safety and legal compliance.
Logistics & Compliance Guide for Laparotomy Drape
Product Overview
A laparotomy drape is a sterile, single-use surgical drape designed for use during abdominal surgical procedures. It provides a sterile barrier to reduce the risk of surgical site infections (SSIs) and ensures controlled access to the surgical field. Drapes are typically made from fluid-resistant materials and may include features such as adhesive borders, fenestrations (pre-cut openings), and antimicrobial coatings.
Regulatory Classification
Laparotomy drapes are classified as medical devices. In the United States, they are typically regulated by the FDA under Class II (moderate risk) and are subject to 510(k) premarket notification requirements. In the European Union, they fall under the Medical Device Regulation (MDR) (EU) 2017/745 and are generally classified as Class I or Class IIa, depending on sterility and intended use. Proper classification ensures compliance with regional regulatory standards.
Sterility and Packaging Requirements
Laparotomy drapes must be supplied sterile and individually packaged in validated sterile barrier systems. Packaging must maintain sterility until the point of use and include clear labeling indicating:
– Sterile status
– Expiration date
– Lot number
– Instructions for use (IFU)
– Manufacturer information
– Symbols per ISO 15223-1
Packaging should be robust enough to withstand transportation stresses while preventing punctures or breaches.
Storage Conditions
Store laparotomy drapes in a clean, dry, temperature-controlled environment:
– Temperature: 15°C to 30°C (59°F to 86°F)
– Relative humidity: Less than 60%
– Away from direct sunlight and heat sources
– On shelves, not directly on the floor
Ensure stock rotation follows FIFO (First In, First Out) principles to prevent expired product use.
Transportation and Distribution
During shipping, ensure:
– Protection from moisture, extreme temperatures, and physical damage
– Use of validated cold chain logistics if required (rare for non-temperature-sensitive drapes)
– Compliance with international shipping standards (e.g., ISTA 3A for parcel delivery)
– Proper documentation, including shipping manifests and customs forms for cross-border shipments
Drapes should remain sealed and undamaged upon arrival at the destination.
Import/Export Compliance
For international trade:
– Verify compliance with destination country regulations (e.g., Health Canada, TGA in Australia, PMDA in Japan)
– Obtain necessary import permits or certificates of free sale
– Ensure labeling is in the local language where required
– Comply with customs documentation, including HS codes (e.g., 6307.90 for other made-up textile articles, or specific medical device codes)
– Maintain records for traceability and audits
Labeling and UDI Requirements
As a regulated medical device, laparotomy drapes must comply with Unique Device Identification (UDI) requirements:
– U.S.: FDA UDI rule – label must include UDI in both human-readable and AIDC (e.g., barcode) format
– EU: MDR requires UDI placement on labels and packaging, with registration in EUDAMED
– Include device name, version/model, manufacturer, sterilization method, and single-use symbol
Clinical and Safety Compliance
- Intended Use: For sterile coverage during laparotomy and other major abdominal surgeries
- Single-Use Only: Do not reprocess or reuse
- Contraindications: Do not use if package is damaged or past expiration
- Adverse Event Reporting: Report any incidents (e.g., drape failure, allergic reactions) to the manufacturer and relevant regulatory authority (e.g., FDA MedWatch, EUDAMED Vigilance)
Environmental and Disposal Considerations
After use, laparotomy drapes are considered biohazardous medical waste. They must be disposed of in accordance with local healthcare waste management regulations:
– Segregate into appropriate waste streams (e.g., red bag waste)
– Follow facility protocols for decontamination and disposal
– Do not dispose of in regular landfill where prohibited
Quality Management System (QMS)
Manufacturers and distributors must maintain a certified Quality Management System compliant with:
– ISO 13485:2016 (Medical devices – Quality management systems)
– FDA Quality System Regulation (21 CFR Part 820)
– EU MDR Annex IX and X requirements
This includes design controls, risk management (ISO 14971), supplier controls, and post-market surveillance.
Training and Staff Awareness
Ensure healthcare staff are trained on:
– Proper handling and opening of sterile packaging
– Correct application techniques to maintain sterility
– Recognition of product defects or packaging breaches
– Reporting procedures for adverse events or device malfunctions
Audit and Traceability
Maintain complete records for full traceability, including:
– Batch/lot tracking from manufacturer to end-user
– Distribution logs
– Complaint and non-conformance records
– Internal and external audit trails
Support recalls swiftly through robust traceability systems.
Conclusion for Sourcing Laparotomy Drapes:
After a thorough evaluation of potential suppliers, product quality, cost-effectiveness, and compliance with medical standards, it is concluded that sourcing laparotomy drapes requires a strategic balance between clinical performance and supply chain reliability. High-quality, sterile, and fluid-resistant drapes that meet ISO and FDA regulations are essential to ensure patient safety and surgical efficacy. Bulk procurement from reputable manufacturers with consistent quality control, sustainable production practices, and timely delivery capabilities offers the best value. Additionally, establishing long-term partnerships with suppliers who provide customization options, responsive customer support, and transparent pricing will enhance operational efficiency in surgical departments. Ultimately, the recommended sourcing strategy supports improved patient outcomes, regulatory compliance, and cost optimization across healthcare facilities.