The global intrauterine insemination (IUI) catheter market is experiencing steady growth, driven by rising infertility rates, increasing awareness of assisted reproductive technologies (ART), and advancements in fertility treatment techniques. According to Grand View Research, the global fertility treatment market was valued at USD 20.5 billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 9.8% from 2024 to 2030, with IUI procedures representing a significant segment of non-invasive ART solutions. As demand for minimally invasive and cost-effective fertility interventions grows, manufacturers of high-precision IUI catheters are playing a pivotal role in improving procedural success rates and patient comfort. This increasing clinical reliance underscores the importance of identifying the top-performing manufacturers contributing to innovation, reliability, and accessibility in reproductive medicine.
Top 5 Intrauterine Insemination Catheter Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
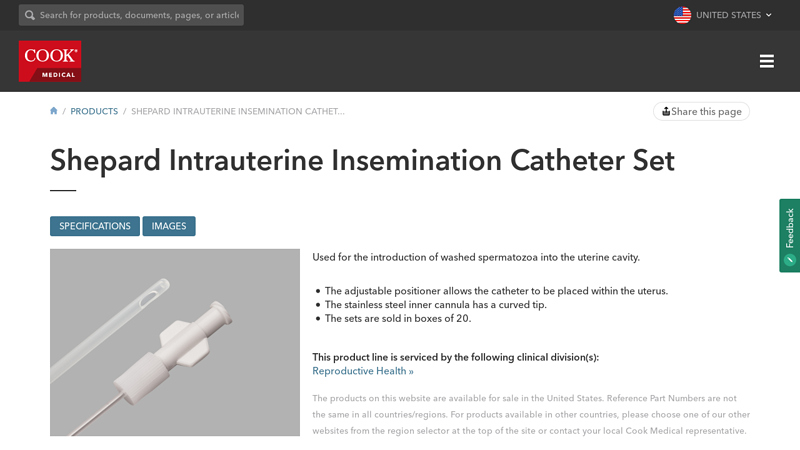
#1 Shepard Intrauterine Insemination Catheter Set
Domain Est. 2003
Website: cookmedical.com
Key Highlights: Used for the introduction of washed spermatozoa into the uterine cavity. The adjustable positioner allows the catheter to be placed within the uterus….
#2 Insemination Catheters For Sale
Domain Est. 2009
Website: ciamedical.com
Key Highlights: 1–4 day delivery 30-day returnsSee our list of insemination catheters and prices below. Order online, call us at (312) 275-5850, or get a 2-hour quote by filling the form on this p…
#3 PM
Domain Est. 2012
Website: prince-medical.com
Key Highlights: The PM-LIFE® IU intrauterine insemination catheter is used to perform insemination during medically assisted reproduction (MAR), by introducing washed and ……
#4 IUI Catheter
Domain Est. 2015
Website: vitavitro.com
Key Highlights: Two catheter lengths to meet different patients’ needs. Smooth and rounded tip for an easy insertion, and to reduce trauma….
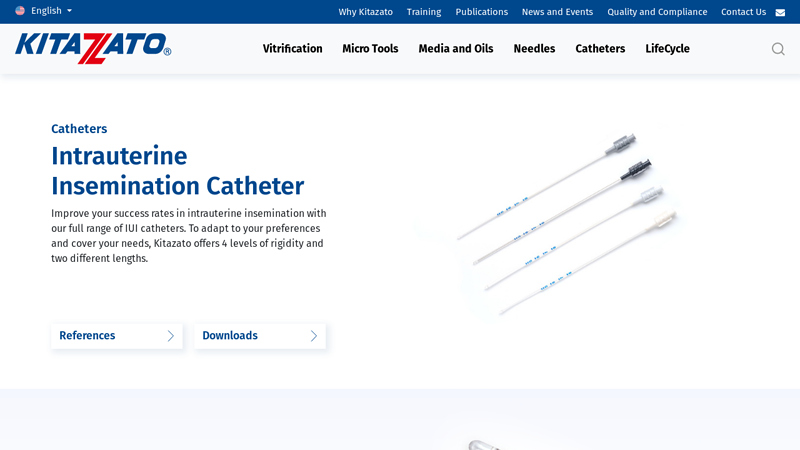
#5 Intrauterine Insemination Catheter
Domain Est. 2020
Website: kitazato-ivf.com
Key Highlights: To adapt to your preferences and cover your needs, Kitazato offers 4 levels of rigidity and 2 different lengths of Intrauterine Insemination Catheters….
Expert Sourcing Insights for Intrauterine Insemination Catheter
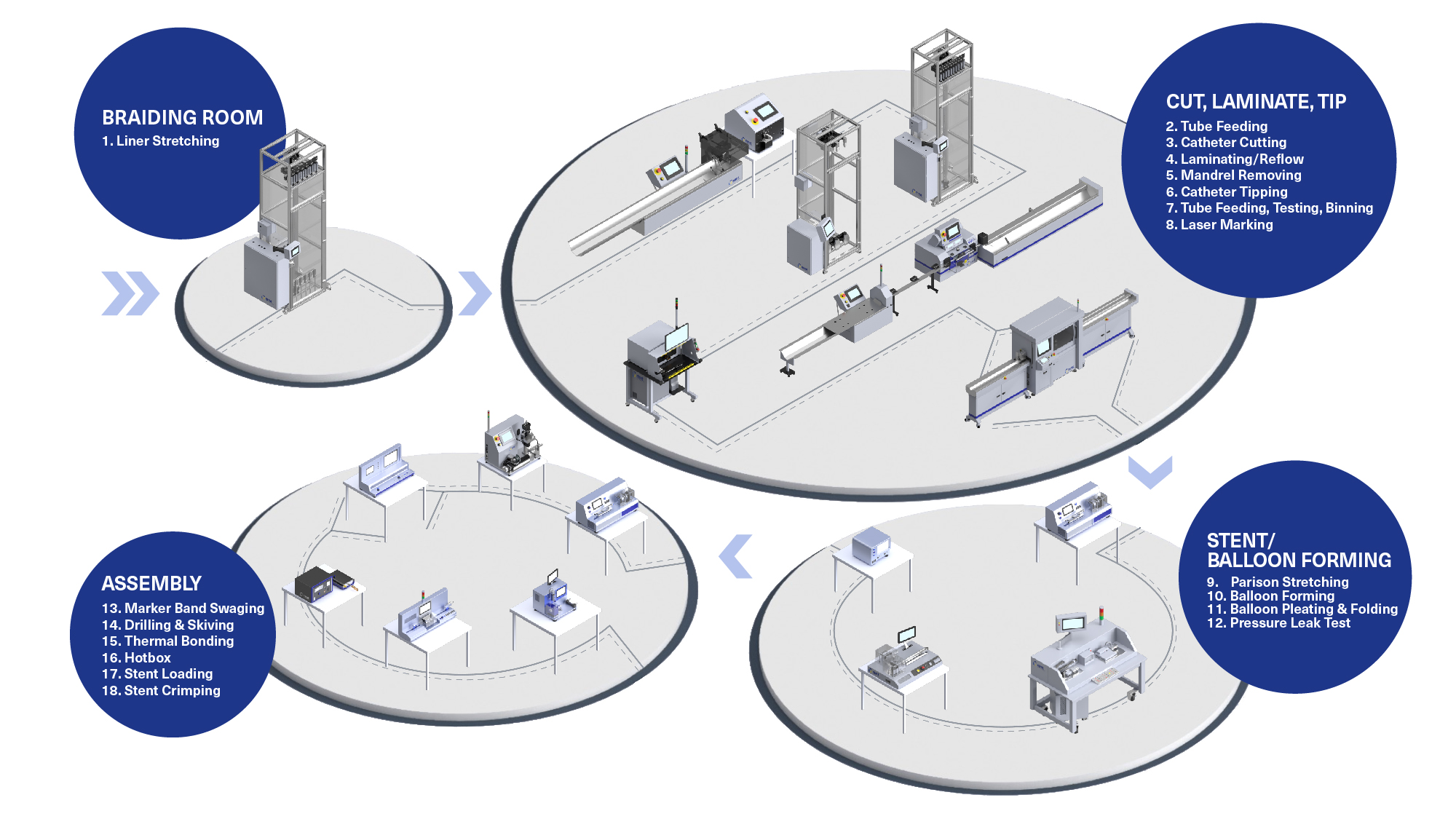
H2: 2026 Market Trends for Intrauterine Insemination (IUI) Catheters
The global market for intrauterine insemination (IUI) catheters is poised for steady growth and transformation by 2026, driven by evolving fertility treatment demands, technological advancements, and shifting demographic and regulatory landscapes. Below is an in-depth analysis of key trends expected to shape the IUI catheter market in 2026.
-
Rising Global Infertility Rates
A primary driver of IUI catheter demand is the increasing prevalence of infertility worldwide. According to the World Health Organization (WHO), approximately 1 in 6 people experience infertility, with rising rates attributed to delayed childbearing, lifestyle factors, and environmental influences. As more individuals and couples seek fertility treatments, the use of cost-effective and minimally invasive options like IUI is expected to increase—directly boosting demand for high-quality IUI catheters. -
Technological Innovations and Product Differentiation
By 2026, manufacturers are anticipated to focus on enhancing catheter design for improved accuracy, patient comfort, and ease of use. Trends include: - Soft-tipped and pre-loaded catheters to minimize uterine trauma and streamline procedures.
- Ultrasound visibility enhancements, such as radiopaque markers, to support real-time placement accuracy.
- Single-use, sterile disposable kits integrating catheters with syringes and collection devices, reducing contamination risks and improving procedural efficiency.
These innovations are expected to differentiate market leaders and support premium pricing models.
-
Expansion of Fertility Clinics in Emerging Markets
Regions such as Asia-Pacific, Latin America, and parts of Africa are witnessing rapid expansion in fertility clinic infrastructure. Countries like India, Brazil, and South Africa are investing in reproductive healthcare, driven by growing middle-class access and supportive government policies. This expansion will increase the penetration of IUI procedures and, by extension, the demand for IUI catheters in previously underserved markets. -
Shift Toward Minimally Invasive and Cost-Effective Treatments
Compared to in vitro fertilization (IVF), IUI remains a more affordable and less invasive fertility option. As healthcare systems and patients prioritize cost-effective treatments—especially in regions with limited insurance coverage for IVF—IUI adoption is expected to grow. This trend will sustain demand for reliable, easy-to-use catheters, particularly in outpatient and primary care fertility settings. -
Regulatory Harmonization and Quality Standards
By 2026, stricter regulatory oversight, particularly from bodies like the U.S. FDA and EU MDR (Medical Device Regulation), will push manufacturers to ensure higher product quality, traceability, and clinical validation. Compliance with ISO 13485 and other international standards will become essential for market access, favoring established players with robust quality systems and R&D capabilities. -
Strategic Partnerships and Market Consolidation
The IUI catheter market is witnessing increased collaboration between medical device manufacturers and fertility clinics or IVF networks. These partnerships facilitate product feedback, clinical training, and distribution efficiency. Additionally, market consolidation—through acquisitions and mergers—is expected to intensify, enabling larger companies to expand portfolios and geographic reach. -
Focus on Patient-Centric Design and Training
With growing attention to patient experience, manufacturers are likely to invest in ergonomic designs and education tools. Training modules for clinicians on optimal catheter use, alongside patient education materials, will become integral to product offerings. This holistic approach can improve success rates and foster brand loyalty. -
Impact of Digital Health Integration
While IUI catheters themselves are physical devices, the broader fertility ecosystem is embracing digital health tools. In 2026, integration with fertility tracking apps, electronic medical records (EMRs), and telehealth platforms may influence catheter selection and usage patterns, especially in clinics adopting end-to-end digital fertility workflows.
Conclusion
By 2026, the IUI catheter market will be shaped by a confluence of medical, technological, and socioeconomic trends. Growth will be fueled by rising infertility rates, demand for affordable fertility solutions, and innovation in device design. Companies that prioritize quality, usability, and global accessibility are likely to gain competitive advantage in this evolving landscape.
Common Pitfalls Sourcing Intrauterine Insemination (IUI) Catheters: Quality and Intellectual Property (IP) Concerns
Sourcing Intrauterine Insemination (IUI) catheters requires careful attention to both product quality and intellectual property (IP) risks. Overlooking these critical areas can lead to patient safety issues, regulatory non-compliance, supply chain disruptions, and significant legal liabilities. Below are key pitfalls to avoid:
Quality-Related Pitfalls
Using Non-Medical Grade Materials
Sourcing catheters made from substandard or non-biocompatible materials poses serious health risks, including inflammation, infection, or allergic reactions. Ensure materials are certified medical grade (e.g., ISO 10993-compliant) and free from cytotoxic substances.
Lack of Sterility Assurance
Failure to verify validated sterilization processes (e.g., ethylene oxide or gamma irradiation) and proper packaging integrity increases the risk of contamination. Always confirm sterility claims with valid test reports and batch certifications.
Inconsistent Manufacturing Standards
Procuring from manufacturers without adherence to ISO 13485 or other medical device quality management systems can result in inconsistent catheter dimensions, tip flexibility, or lubrication—factors critical for successful insemination and patient comfort.
Insufficient Regulatory Compliance
Sourcing catheters without proper regulatory clearance (e.g., FDA 510(k), CE Marking) may lead to import bans, product recalls, or legal penalties. Verify that devices meet the regulatory requirements of the target market.
Poor Tip Design or Performance
Low-quality catheters may have poorly designed tips that increase the risk of cervical trauma, failed embryo deposition, or catheter breakage. Evaluate clinical feedback and performance data before finalizing suppliers.
Intellectual Property (IP)-Related Pitfalls
Sourcing from IP-Infringing Suppliers
Procuring catheters that copy patented designs (e.g., specific tip configurations, loading mechanisms) exposes buyers to infringement claims. Conduct due diligence to ensure the supplier owns or is licensed to use the technology.
Lack of IP Documentation Review
Failing to request and review IP ownership documentation, licenses, or freedom-to-operate (FTO) opinions leaves buyers vulnerable to litigation. Insist on transparency regarding the supplier’s IP position.
OEM Manufacturing Without Licensing
Engaging original equipment manufacturers (OEMs) that produce patented designs without proper licensing can result in shared liability. Confirm that any OEM arrangement includes valid IP rights.
Counterfeit or Grey Market Products
Purchasing through unauthorized distributors increases the risk of counterfeit devices that not only lack quality controls but also infringe on IP rights. Use only authorized and traceable supply channels.
Inadequate Contracts Addressing IP
Supplier agreements that omit clear IP clauses (e.g., indemnification, ownership of improvements, infringement liabilities) can leave buyers exposed. Ensure contracts explicitly address IP responsibilities and protections.
By proactively addressing these quality and IP pitfalls, healthcare providers and distributors can ensure the safe, legal, and reliable sourcing of IUI catheters.

Logistics & Compliance Guide for Intrauterine Insemination (IUI) Catheter
Regulatory Classification and Approvals
Intrauterine Insemination (IUI) catheters are classified as medical devices and are subject to regulatory oversight depending on the region of use. In the United States, the Food and Drug Administration (FDA) typically classifies IUI catheters as Class II medical devices under 21 CFR 884.4440 (Spermatozoa processing and reproductive tissue handling devices). This classification requires compliance with premarket notification (510(k)) clearance, adherence to Quality System Regulation (QSR) 21 CFR Part 820, and proper labeling.
In the European Union, IUI catheters must comply with the Medical Devices Regulation (MDR) (EU) 2017/745. They are generally classified as Class IIa devices, requiring conformity assessment by a Notified Body. Manufacturers must establish a Quality Management System (QMS) compliant with ISO 13485, prepare a Technical File, and affix the CE mark before placing the device on the market.
Other regions such as Canada (under Health Canada’s Medical Devices Regulations), Australia (TGA), and Japan (PMDA) have their own regulatory frameworks. It is essential to verify local requirements before distribution.
Labeling and Packaging Requirements
IUI catheters must be labeled in accordance with applicable regional regulations. Labels should include:
- Device name and intended use (e.g., “For intrauterine insemination procedures”)
- Manufacturer name, address, and contact information
- Unique Device Identifier (UDI) as required by FDA and EU MDR
- Lot number and expiration date
- Sterile and single-use statements
- Instructions for use (IFU), including contraindications, warnings, and precautions
- Symbols per ISO 15223-1 (e.g., sterile, single-use, do not reuse)
Packaging must maintain sterility throughout distribution and storage. Primary packaging should be validated to preserve microbial barrier integrity. Secondary packaging should protect against physical damage and environmental factors during transport.
Sterility and Biocompatibility
IUI catheters are provided sterile and intended for single use only. Sterilization methods commonly include ethylene oxide (EtO) or gamma irradiation. The chosen method must be validated according to ISO 11135 (for EtO) or ISO 11137 (for radiation), and sterility assurance level (SAL) must be 10⁻⁶.
Biocompatibility must be assessed per ISO 10993-1, including evaluations for cytotoxicity, sensitization, irritation, and systemic toxicity. Given the device’s brief contact with mucosal tissue, testing typically includes short-term exposure endpoints.
Storage and Transportation
IUI catheters must be stored and transported under controlled conditions to maintain sterility and material integrity. Recommended storage conditions generally include:
- Temperature: 15°C to 30°C (59°F to 86°F)
- Relative humidity: 30% to 70%
- Protection from direct sunlight and moisture
Transportation should use validated packaging systems that protect against shock, vibration, compression, and extreme temperatures. Cold chain considerations are typically not required unless specified by the manufacturer.
Supply Chain and Distribution Controls
Distribution of IUI catheters requires strict chain-of-custody controls. Distributors and importers must comply with local regulations and maintain proper documentation, including:
- Validated shipping and storage protocols
- Temperature monitoring (if applicable)
- Traceability records (UDI implementation)
- Proof of regulatory compliance (e.g., CE certificate, FDA listing)
Manufacturers should conduct supplier audits and ensure all parties in the supply chain are trained on handling sterile medical devices.
Post-Market Surveillance and Vigilance Reporting
Manufacturers are required to implement a post-market surveillance (PMS) system to monitor device performance and detect adverse events. Under EU MDR, a Post-Market Surveillance Plan and Periodic Safety Update Report (PSUR) are mandatory for Class IIa devices.
In the U.S., medical device reports (MDRs) must be submitted to the FDA for incidents involving death, serious injury, or malfunction that could cause harm. A robust complaint handling system aligned with 21 CFR 820.198 or ISO 13485 is essential.
Environmental and Disposal Compliance
IUI catheters are single-use devices and must be disposed of according to biohazardous waste regulations after use. Disposal protocols should align with local healthcare waste management standards (e.g., OSHA, EPA in the U.S.; EU directives on waste).
Unused or expired devices should be returned to the manufacturer or disposed of per environmental compliance guidelines. Packaging materials should be minimized and designed for recyclability where feasible.
Training and User Competency
Healthcare providers using IUI catheters must receive appropriate training on device handling, aseptic technique, and procedure protocols. Manufacturers should provide clear Instructions for Use (IFU) and support educational initiatives. Competency assessments may be required by clinics or regulatory bodies to ensure patient safety.
Conclusion:
In sourcing an intrauterine insemination (IUI) catheter, it is essential to prioritize quality, safety, and clinical efficacy. The selection process should involve a thorough evaluation of product specifications, including flexibility, tip design, and ease of use, to ensure minimal discomfort and optimal sperm deposition. Compliance with international regulatory standards (such as ISO 13485 and FDA clearance) is critical to guarantee device safety and performance. Additionally, supplier reliability, consistency in supply, and cost-effectiveness must be balanced without compromising on clinical outcomes. By conducting comprehensive market research, engaging with clinicians, and assessing both technical and logistical factors, healthcare providers can identify a suitable IUI catheter that supports successful fertility treatments while meeting operational and patient care requirements.