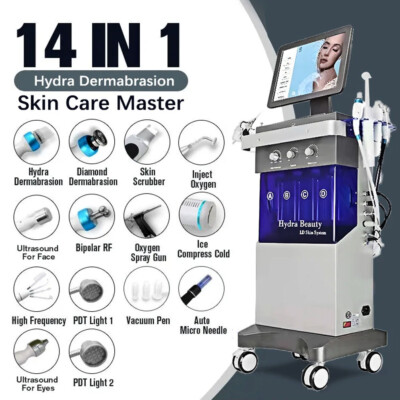
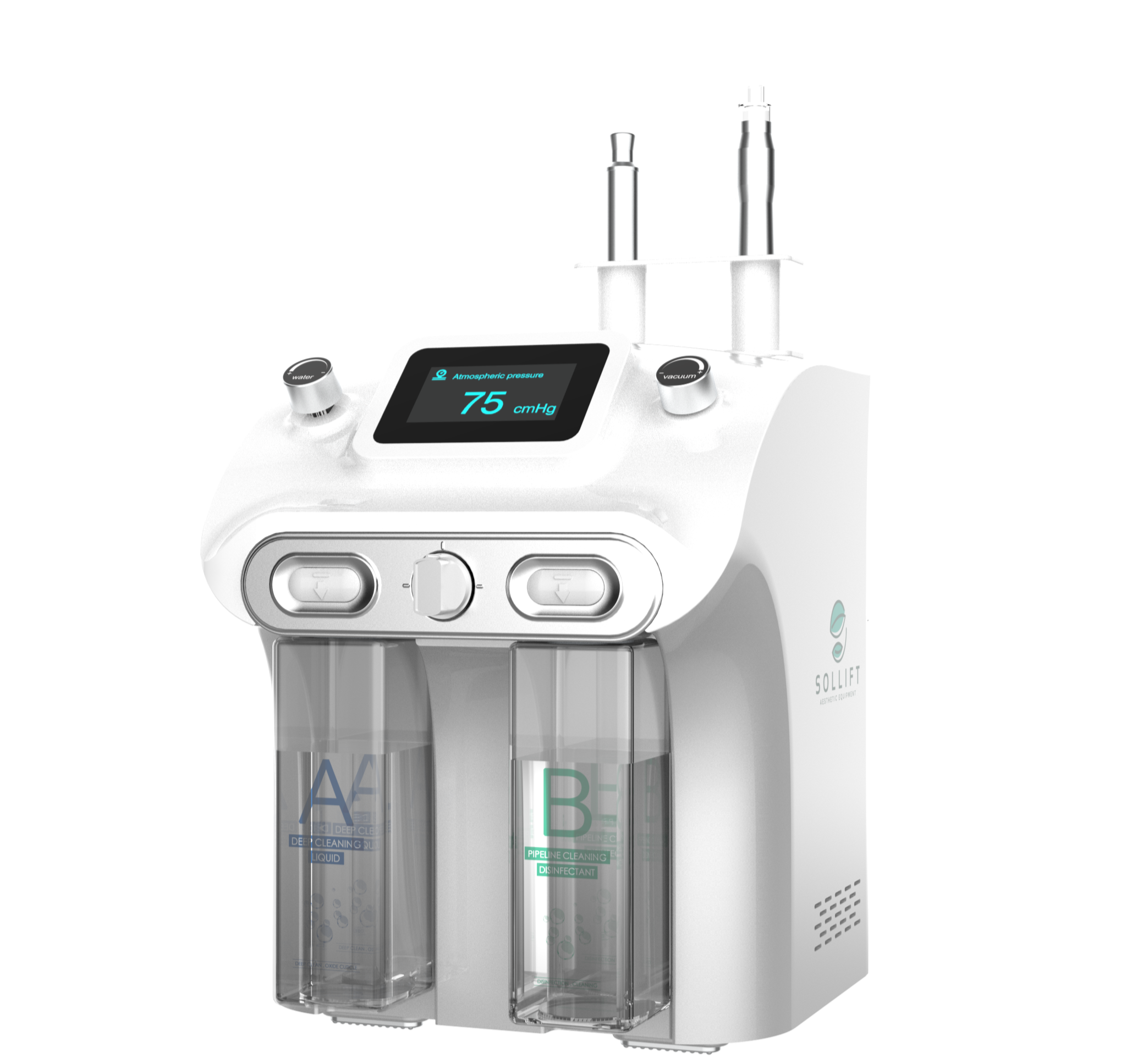
The global dermal repair and skincare treatment market is experiencing robust growth, driven by rising consumer demand for non-invasive aesthetic procedures. According to Mordor Intelligence, the skincare devices market was valued at USD 7.2 billion in 2023 and is projected to grow at a CAGR of over 9.5% through 2029, with hydro dermabrasion emerging as one of the fastest-growing segments due to its effectiveness in exfoliation, hydration, and acne treatment. This surge in demand has catalyzed innovation and competition among manufacturers, leading to advancements in multi-functional, medical-grade hydro dermabrasion systems. As clinics and spas increasingly adopt these devices to meet patient expectations for visible, immediate results, selecting the right manufacturer becomes critical. Based on performance metrics, technological integration, global distribution, and compliance with medical device regulations, we’ve identified the top 7 hydro dermabrasion machine manufacturers shaping the future of professional skincare solutions.
Top 7 Hydro Dermabrasion Machine Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Professional Hydro Dermabrasion Machines for Spas & Clinics
Domain Est. 2003
Website: surebeauty.com
Key Highlights: Shop professional hydro dermabrasion machines for salons. Deep cleanse, hydrate, and rejuvenate skin with non-invasive, spa-grade technology….
#2 DiamondGlow®
Domain Est. 2004
Website: diamondglow.com
Key Highlights: The DiamondGlow® device is a general dermabrasion device that gently removes the top layer of skin and delivers topical cosmetic serums onto the skin….
#3 Professional Hydradermabrasion Machines
Domain Est. 2010
Website: seaheartbj.com
Key Highlights: Shop professional hydradermabrasion and hydrofacial machines. Powerful hydra dermabrasion systems for salons, spas, and clinics….
#4 DermaJEM
Domain Est. 2020
Website: dermajem.com
Key Highlights: 2–5 day deliveryShop All · Hydro Devices · Aesthetic Laser · Face · Body · Hair Removal · Accessories. 1 on 1 training with any machine purchase. Shop now….
#5 Buy Hydra Facial Microdermabrasion Machines Online In US
Domain Est. 2021
#6 Hydra Dermabrasion Facial Machine Professional
Domain Est. 2023
#7 Hydrafacial
Domain Est. 2005
Website: hydrafacial.com
Key Highlights: Hydrafacial treatments are the ultimate solution for improving the look of dullness, fine lines and pores, promoting smoother and more radiant-looking skin….
Expert Sourcing Insights for Hydro Dermabrasion Machine
2026 Market Trends for Hydro Dermabrasion Machines
The global market for hydro dermabrasion machines is poised for notable expansion by 2026, driven by rising consumer demand for non-invasive skincare solutions, technological advancements, and growing awareness of skin health. This analysis explores key market trends shaping the hydro dermabrasion industry in 2026, focusing on innovation, consumer behavior, regional dynamics, and competitive landscape.
Technological Innovation and Device Sophistication
In 2026, hydro dermabrasion machines are expected to feature enhanced technological integration, including smart sensors, AI-assisted skin analysis, and IoT connectivity. Leading manufacturers are incorporating real-time skin diagnostics into devices, allowing practitioners to customize treatments based on individual skin conditions. Touchscreen interfaces, automated pressure control, and multi-functional modules (such as infusion of serums and LED therapy) are becoming standard, increasing the appeal of these systems in both clinical and spa environments.
Portable and at-home hydro dermabrasion devices are also gaining traction, with improved ergonomics and safety features making them viable for consumer use. These compact models often sync with mobile apps to track skin progress, offering personalized skincare regimens and boosting user engagement.
Rising Demand for Non-Invasive Aesthetic Procedures
The preference for minimally invasive or non-invasive cosmetic treatments continues to grow, with hydro dermabrasion emerging as a popular alternative to chemical peels and microdermabrasion. Consumers are increasingly seeking treatments that require no downtime, minimal discomfort, and deliver visible results. Hydro dermabrasion meets these criteria by combining exfoliation, extraction, and hydration in a single session, making it ideal for addressing concerns like acne, dullness, enlarged pores, and early signs of aging.
This demand is amplified by the influence of social media and digital beauty communities, where clear, glowing skin is highly valued. As a result, aesthetic clinics, dermatology practices, and medi-spas are investing in hydro dermabrasion machines to expand service offerings and attract younger demographics.
Expansion in Emerging Markets
Asia-Pacific, particularly countries like China, India, and South Korea, is projected to be the fastest-growing region for hydro dermabrasion machines in 2026. Increasing disposable incomes, urbanization, and a strong cultural emphasis on skincare are fueling market growth. South Korea’s influence on global beauty trends, combined with China’s expanding medical aesthetics sector, is driving adoption in both professional and home-use segments.
Latin America and the Middle East are also witnessing rising demand, with growing investments in medical tourism and luxury wellness centers. These regions are becoming key targets for international manufacturers seeking to expand their global footprint.
Integration with Premium Skincare Brands
A significant trend in 2026 is the strategic partnerships between hydro dermabrasion device manufacturers and premium skincare brands. Devices are increasingly designed to work with branded serums and treatment solutions, creating a closed-loop ecosystem that enhances treatment efficacy and encourages product sales. These collaborations not only improve clinical outcomes but also increase customer loyalty and generate recurring revenue through consumables.
Clinics offering branded hydro facial treatments—such as those using SkinMedica or other dermatologist-recommended products—are seeing higher client retention and premium pricing power.
Regulatory and Safety Developments
As the market grows, regulatory scrutiny is intensifying, especially for at-home devices. In 2026, stricter safety standards and certification requirements are expected in regions like the EU and North America. Manufacturers are investing in clinical testing and compliance to meet FDA, CE, and other regulatory benchmarks, ensuring consumer safety and building trust.
Additionally, professional training and certification programs for operators are becoming more prevalent, reinforcing the legitimacy of hydro dermabrasion as a clinical-grade treatment.
Competitive Landscape and Market Consolidation
The hydro dermabrasion market is becoming increasingly competitive, with established players such as Edge Systems (HydroFacial), Image Skincare, and Dermalogica dominating the professional segment. However, new entrants from China and South Korea are introducing cost-effective alternatives, increasing price competition.
In response, major companies are focusing on R&D, brand differentiation, and global distribution networks. Mergers and acquisitions are likely to rise as companies seek to consolidate market share and expand into adjacent skincare technologies.
Conclusion
By 2026, the hydro dermabrasion machine market will be characterized by technological sophistication, expanding consumer access, and strong growth in emerging economies. The convergence of skincare science, digital health, and aesthetic wellness is positioning hydro dermabrasion as a cornerstone treatment in modern dermatology and beauty regimens. As innovation continues and consumer expectations evolve, stakeholders across the value chain—from manufacturers to skincare providers—will need to adapt strategically to capitalize on these dynamic trends.

Common Pitfalls When Sourcing a Hydro Dermabrasion Machine (Quality and IP)
Sourcing a hydro dermabrasion machine involves significant investment and risk, particularly concerning product quality and intellectual property (IP) protection. Overlooking critical aspects can lead to operational failures, legal disputes, and reputational damage. Below are key pitfalls to avoid:
Poor Build Quality and Substandard Components
Many suppliers—especially those offering extremely low prices—cut corners by using inferior materials and untested components. This can result in inconsistent water and vacuum pressure, unreliable exfoliation performance, and frequent mechanical breakdowns. Machines with poor sealing or low-grade pumps are prone to leaks and reduced lifespan, increasing maintenance costs and downtime. Always request third-party certifications (such as ISO 13485 or CE for medical devices) and conduct factory audits or request product samples before committing.
Lack of IP Verification and Risk of Infringement
A major concern when sourcing hydro dermabrasion machines, particularly from regions with lax IP enforcement, is the risk of purchasing counterfeit or cloned devices. Some manufacturers reverse-engineer patented technology without authorization, exposing buyers to legal liability. Before purchasing, verify that the supplier owns the design rights or holds valid licensing agreements. Request proof of patents, trademarks, and regulatory approvals. Failure to do so may result in customs seizures, lawsuits, or being forced to discontinue use of the equipment.
Inadequate Regulatory Compliance
Hydro dermabrasion machines may be classified as medical or cosmetic devices depending on the market. Sourcing a machine that does not meet local regulatory standards (e.g., FDA in the U.S., Health Canada, or EU MDR) can prevent legal operation or sale. Ensure the supplier provides full documentation, including technical files, conformity assessments, and language-specific user manuals. Non-compliant machines may also lack essential safety features, posing risks to both practitioners and clients.
Hidden Costs and Lack of After-Sales Support
Low initial pricing often masks additional expenses such as proprietary handpieces, replacement filters, or software subscriptions. Additionally, some suppliers offer little or no technical support, training, or warranty service—especially if based overseas. This can leave you stranded when issues arise. Confirm the availability of spare parts, service technicians, and software updates, and consider the total cost of ownership, not just the upfront price.
Misleading Marketing and Unverified Performance Claims
Some suppliers exaggerate machine capabilities, such as suction power, treatment versatility, or compatibility with various serums. Without independent testing or clinical validation, these claims may be unsubstantiated. Request clinical studies, demo units, or references from existing clients to verify performance. Be cautious of generic, white-labeled machines rebranded with premium features that don’t exist in practice.
By carefully evaluating quality standards, verifying IP rights, ensuring regulatory compliance, and assessing long-term support, you can avoid costly mistakes and invest in a reliable, legally sound hydro dermabrasion solution.
Logistics & Compliance Guide for Hydro Dermabrasion Machine
This guide outlines the essential logistics and regulatory compliance considerations for the import, distribution, and operation of Hydro Dermabrasion Machines. Adherence to these guidelines ensures legal operation, patient safety, and smooth supply chain management.
Regulatory Classification & Approvals
Hydro Dermabrasion Machines are typically classified as medical devices in most jurisdictions. The specific classification (e.g., Class I, IIa, IIb) depends on design, function, and risk profile.
- FDA (USA): Requires clearance or approval under 510(k) or De Novo pathway. The device must comply with Quality System Regulation (QSR/21 CFR Part 820). Obtain FDA Establishment Registration and Device Listing.
- CE Marking (EU/UK): Must comply with the Medical Device Regulation (MDR) (EU) 2017/745. Requires conformity assessment (often involving a Notified Body), Technical Documentation, Declaration of Conformity (DoC), and registration in EUDAMED.
- Health Canada: Requires a Medical Device Licence (MDL) under the Medical Devices Regulations (SOR/98-282). Classification ranges from Class I to IV.
- TGA (Australia): Requires inclusion in the Australian Register of Therapeutic Goods (ARTG). Classification follows the EU system (Class I to III).
- Other Markets: Research specific requirements for target countries (e.g., ANVISA in Brazil, PMDA in Japan, NMPA in China). Obtain necessary local authorizations.
Import & Export Documentation
Ensure all international shipments include accurate and complete documentation to prevent customs delays or seizures.
- Commercial Invoice: Detailed description of goods (including HS Code), value, currency, Incoterms® rules, buyer/seller information.
- Packing List: Itemized list of contents per package, weights, dimensions, and markings.
- Bill of Lading/Air Waybill: Contract of carriage and receipt for goods.
- Certificate of Origin: May be required for preferential tariffs or import restrictions.
- Regulatory Documents: Copies of FDA 510(k), CE Certificate of Conformity, local market authorizations, or import permits as required by the destination country.
- Safety Data Sheets (SDS): Required if the machine uses or contains hazardous substances (e.g., certain cleaning solutions).
Shipping & Handling Requirements
Proper packaging and handling are critical to prevent damage during transit.
- Packaging: Use robust, manufacturer-approved packaging with adequate cushioning (foam, bubble wrap). Include desiccants if sensitive to moisture. Clearly label with “Fragile,” “This Side Up,” and “Protect from Moisture.”
- Environmental Controls: Ship within specified temperature and humidity ranges if required by manufacturer specifications. Avoid extreme conditions.
- Handling: Train personnel on safe lifting and handling procedures. Use appropriate equipment (e.g., dollies, forklifts) for heavy units.
- Insurance: Secure comprehensive cargo insurance covering loss, damage, and theft during transit.
Customs Clearance & Duties
Navigate import procedures efficiently by understanding applicable tariffs and regulations.
- HS Code Classification: Accurately classify the machine using the Harmonized System (HS) code. Common codes may fall under 9018 (instruments and appliances used in medical, surgical…), but verify precisely.
- Import Duties & Taxes: Calculate applicable import duties, VAT, GST, or other local taxes based on HS code, value, and country of origin. Utilize trade agreements if eligible.
- Customs Broker: Engage a licensed customs broker familiar with medical device regulations in the destination country to facilitate clearance.
- Prohibited/Restricted Items: Confirm the device is not subject to import bans or special restrictions (e.g., dual-use items, controlled components).
Installation, Training, and Maintenance
Ensure safe and effective use through proper setup and support.
- Installation: Follow manufacturer protocols. Verify site requirements (power, water supply, drainage, ventilation) are met. Calibration may be required.
- Operator Training: Provide comprehensive training for all users on operation, safety procedures, contraindications, infection control, and emergency shutdown.
- Maintenance & Servicing: Adhere to the manufacturer’s recommended maintenance schedule. Use only authorized service personnel and genuine parts. Maintain detailed service logs.
- Technical Support: Establish access to reliable technical support and spare parts supply chain.
Safety, Infection Control, and Environmental Compliance
Prioritize user and patient safety and environmental responsibility.
- Electrical Safety: Ensure compliance with standards like IEC 60601-1. Regular electrical safety testing may be required.
- Infection Control: Implement strict protocols for cleaning, disinfection, and sterilization of reusable tips and accessories between patients, following manufacturer and local health authority guidelines. Manage single-use items appropriately.
- Waste Disposal: Dispose of used tips, filters, and contaminated waste according to local biomedical or hazardous waste regulations. Never dispose of down drains if prohibited.
- Environmental Regulations: Comply with regulations regarding the disposal of electronic waste (e-waste/WEEE) and any hazardous substances within the device (e.g., batteries, certain plastics).
Record Keeping and Audits
Maintain thorough documentation for compliance and traceability.
- Device Tracking: Implement a system to track device serial numbers, locations, and service history.
- Regulatory Documentation: Maintain up-to-date copies of all regulatory approvals, certificates, and declarations.
- Training Records: Document all operator training sessions and certifications.
- Maintenance Logs: Keep detailed records of all maintenance, repairs, and calibrations.
- Adverse Event Reporting: Establish procedures for reporting device malfunctions or adverse events to relevant authorities (e.g., FDA MedWatch, EUDAMED) as mandated.
- Audit Preparedness: Be ready for internal or regulatory audits by ensuring all records are organized and accessible.
Disclaimer: Regulations vary significantly by country and are subject to change. Consult with legal, regulatory, and logistics experts specializing in medical devices for your target markets before import or distribution.
Conclusion for Sourcing a Hydro Dermabrasion Machine
In conclusion, sourcing a hydro dermabrasion machine requires careful consideration of treatment efficacy, device functionality, safety features, brand reputation, and long-term support. These advanced skincare systems offer non-invasive, multi-functional benefits—such as exfoliation, hydration, extraction, and infusion—that meet the growing demand for professional-grade facial treatments. When selecting a machine, prioritize FDA-cleared or CE-certified devices with proven technologies, intuitive user interfaces, and customizable treatment options to cater to diverse skin types and conditions.
Additionally, evaluating warranty terms, training programs, and maintenance support is essential for ensuring smooth integration into your practice and maximizing return on investment. Whether purchasing new or refurbished, sourcing from reputable suppliers or manufacturers with strong customer service will enhance reliability and client satisfaction. Ultimately, a well-chosen hydro dermabrasion machine can elevate your service offerings, boost client retention, and strengthen your business’s competitiveness in the aesthetic industry.