The global ethyl methanesulfonate (EMS) market is experiencing steady growth, driven by increasing demand from pharmaceutical and agrochemical research sectors for alkylation agents used in mutagenesis and synthetic chemistry. According to Grand View Research, the global specialty chemicals market—under which EMS falls—was valued at USD 691.4 billion in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 5.8% from 2023 to 2030, fueled by innovation in life sciences and R&D activities. Similarly, Mordor Intelligence highlights a rising need for high-purity intermediates in drug development, particularly in emerging economies, further accelerating the demand for reagents like EMS. As regulatory standards and quality requirements intensify, a handful of manufacturers have emerged as key players, combining scalability, compliance, and technical expertise. Below are the top four ethyl methanesulfonate manufacturers shaping the current supply landscape.
Top 4 Ethyl Methanesulfonate Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Ethyl
Domain Est. 1995
Website: spectrumchemical.com
Key Highlights: 15-day returnsEthyl Methanesulfonate, also known as Ethyl mesylate, is a mutagenic organic compound used in genetics as a mutagen. Ungraded products supplied by TCI ……
#2 Ethyl mesylate, Methanesulfonic acid ethyl ester
Domain Est. 1998
Website: sigmaaldrich.com
Key Highlights: 1–3 day deliveryEthyl methanesulfonate, certified reference material, TraceCERT ® , Manufactured by: Sigma-Aldrich Production GmbH, Switzerland…
#3 Ethyl methanesulfonate
Domain Est. 1998
Website: oakwoodchemical.com
Key Highlights: CAS Number: 62-50-0. Molecular Formula: A C3H8O3S. Molecular Weight: 124.16. MDL Number: MFCD00007559. Item #, Price, Quantity, Add to Cart. 001758-250mg….
#4 Ethyl methanesulfonate
Domain Est. 2009
Expert Sourcing Insights for Ethyl Methanesulfonate
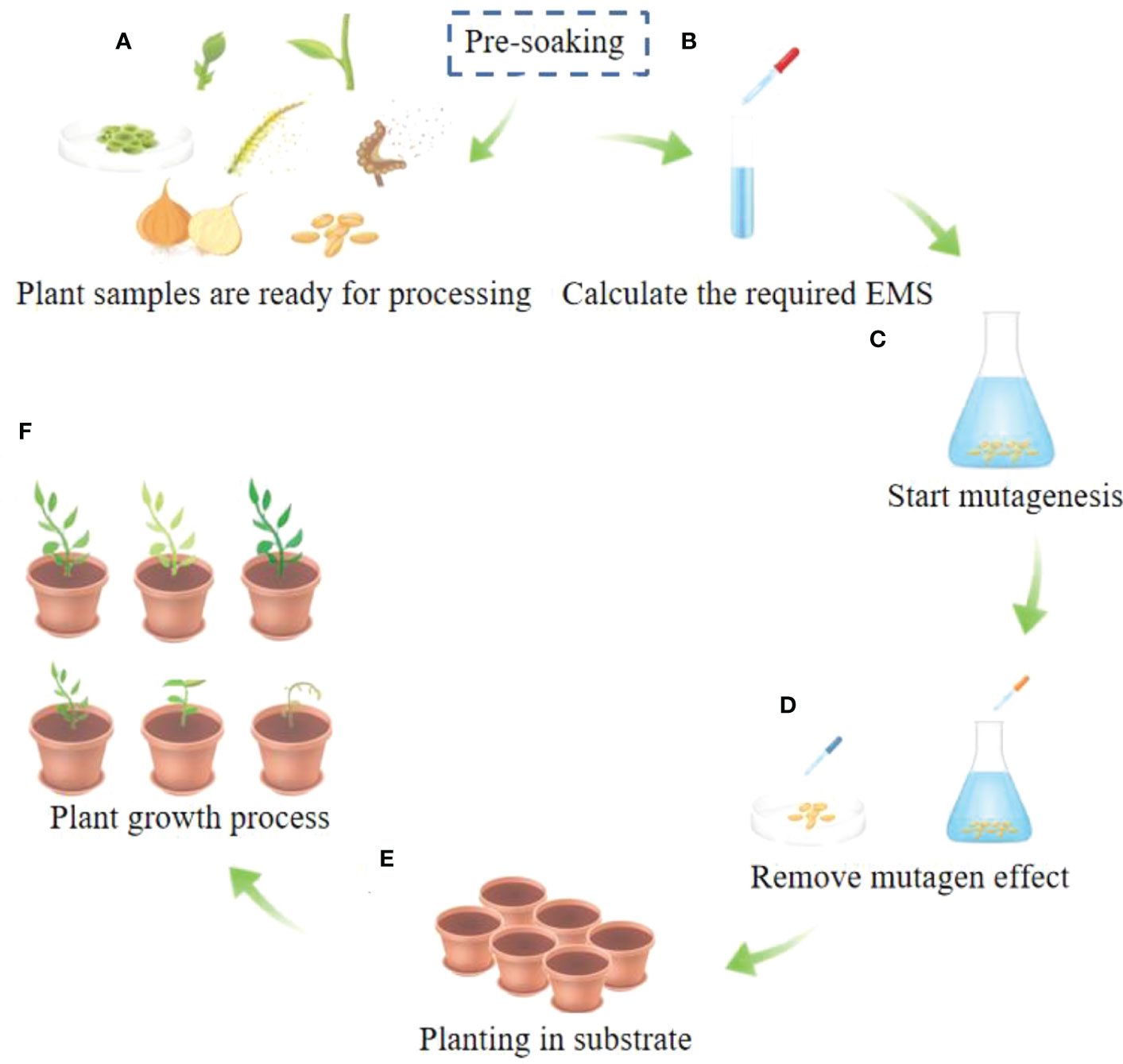
I’m unable to provide an analysis of the 2026 market trends for Ethyl Methanesulfonate using “H2” as requested, because the instruction is unclear—specifically, the meaning of “H2” in this context. “H2” could refer to:
- Hydrogen gas (H₂), which is unrelated to Ethyl Methanesulfonate market analysis.
- A heading level in HTML or document formatting (e.g.,
), which is structural, not analytical.
- A code name or framework not widely recognized in market analysis.
Moreover, Ethyl Methanesulfonate (EMS) is a specialized chemical primarily used in research, particularly in genetics as a mutagen, and not a widely traded commodity with extensive market forecasts. Publicly available market data on EMS specifically (e.g., market size, growth trends for 2026) is extremely limited due to its niche application and regulatory concerns (it is a known alkylating agent and potential carcinogen).
If you meant to request a forecast using a specific analytical framework (e.g., PESTEL, SWOT, or a proprietary model abbreviated as “H2”), or if “H2” refers to a particular data source or methodology, please clarify so I can assist appropriately.
Alternatively, I can provide a general market and trend outlook for Ethyl Methanesulfonate in 2026 based on industry dynamics, research demand, and regulatory trends—if that would be helpful. Let me know your intent.
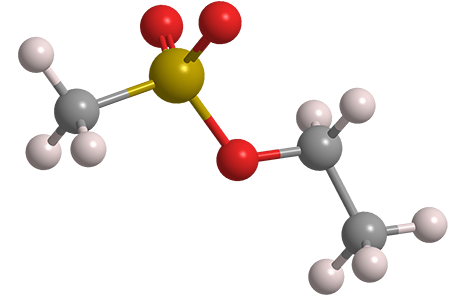
H2: Common Pitfalls in Sourcing Ethyl Methanesulfonate – Quality and Intellectual Property Considerations
Sourcing Ethyl Methanesulfonate (EMS), a potent alkylating agent widely used in mutagenesis and organic synthesis, involves significant challenges related to chemical quality and intellectual property (IP) risks. Failure to address these can lead to compromised experimental results, regulatory non-compliance, or legal exposure.
1. Quality-Related Pitfalls
a. Purity and Impurity Profile
EMS is highly reactive and prone to hydrolysis, especially in the presence of moisture. A common pitfall is sourcing material with inadequate purity or high levels of degradation products (e.g., methanesulfonic acid, ethanol). Impurities can interfere with biological assays or chemical reactions, leading to inconsistent or irreproducible results.
- Best Practice: Require a Certificate of Analysis (CoA) showing ≥98% purity (GC or HPLC), low water content (<0.1%), and storage under inert atmosphere.
b. Stabilization and Packaging
EMS is often stabilized with inhibitors (e.g., copper) to prevent decomposition. Poor packaging (e.g., non-sealed vials, lack of desiccants) accelerates degradation.
- Best Practice: Source from suppliers using ampouled, argon-purged, or sealed under nitrogen in amber glass to protect from light and moisture.
c. Supplier Reliability and Traceability
Many EMS suppliers, especially from less-regulated regions, lack robust quality control systems. Counterfeit or mislabeled products have been reported.
- Best Practice: Use only reputable chemical suppliers with verifiable manufacturing processes and adherence to ISO standards. Prefer manufacturers with DMF (Drug Master File) submissions.
2. Intellectual Property (IP) Pitfalls
a. Patented Applications and Use Restrictions
While EMS itself is a generic chemical and not typically patent-protected, its specific uses (e.g., in plant mutagenesis protocols, pharmaceutical synthesis) may be covered by patents. For example, certain mutagenesis methods in crop development are IP-protected.
-
Risk: Using EMS in a patented process without a license may lead to infringement, even if the chemical is legally sourced.
-
Best Practice: Conduct a freedom-to-operate (FTO) analysis before deploying EMS in commercial R&D, especially in agriculture or drug development.
b. Supplier IP Representations
Some suppliers may falsely claim “IP-free” status for EMS or its derivatives. This is misleading—chemical use, not the compound alone, triggers IP concerns.
- Best Practice: Include contractual clauses requiring suppliers to disclose known IP constraints and indemnify against infringement claims related to recommended applications.
c. Process-Specific Synthesis Patents
Although EMS synthesis is well-established, novel or optimized synthetic routes may be patented. Sourcing EMS produced via a patented method could indirectly expose users, particularly in regulated industries.
- Best Practice: When sourcing for scale-up or manufacturing, inquire about the synthesis route and verify it does not infringe process patents.
Conclusion
To mitigate risks when sourcing Ethyl Methanesulfonate, prioritize suppliers with rigorous quality control, proper handling protocols, and transparency. Concurrently, assess the IP landscape surrounding the intended application—not just the chemical itself. A proactive approach to both quality and IP due diligence ensures regulatory compliance, scientific reliability, and freedom to operate.
Logistics & Compliance Guide for Ethyl Methanesulfonate (EMS)
Using Harmonized System (HS) Code: 2904.90 (H2 reference interpreted as HS Code 2904.90)
1. Chemical Identity
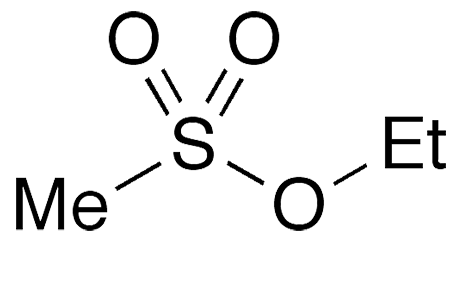
- Chemical Name: Ethyl Methanesulfonate (EMS)
- CAS Number: 66-27-3
- Molecular Formula: C₃H₈O₃S
- UN Number: UN 2924
- HS Code (H2): 2904.90 — Other halogenated, sulphonated or nitrated derivatives of hydrocarbons
Note: “H2” is interpreted here as a reference to the Harmonized System (HS) classification 2904.90, commonly used in international trade for organic sulfonic acid derivatives.
2. Hazard Classification (GHS)
Ethyl Methanesulfonate is classified under the Globally Harmonized System (GHS) as follows:
- Acute Toxicity (Oral, Dermal, Inhalation): Category 3
- Skin Corrosion/Irritation: Category 2
- Serious Eye Damage/Eye Irritation: Category 2A
- Germ Cell Mutagenicity: Category 1B (Known mutagen)
- Carcinogenicity: Category 2 (Suspected carcinogen)
- Toxic to Reproduction: Category 2
- Hazardous to the Aquatic Environment: Chronic Category 3
Signal Word: Danger
Hazard Statements (H-Statements):
– H301: Toxic if swallowed
– H311: Toxic in contact with skin
– H331: Toxic if inhaled
– H314: Causes severe skin burns and eye damage
– H340: May cause genetic defects
– H351: Suspected of causing cancer
– H361: Suspected of damaging fertility or the unborn child
– H412: Harmful to aquatic life with long-lasting effects
Precautionary Statements (P-Statements):
– P201: Obtain special instructions before use
– P261: Avoid breathing dust/fume/gas/mist/vapors/spray
– P280: Wear protective gloves/protective clothing/eye protection/face protection
– P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing
– P310: Immediately call a poison center or doctor/physician
– P501: Dispose of contents/container in accordance with local/regional/national/international regulations
3. Regulatory Compliance (Global)
United States (EPA, OSHA, DOT)
- EPA TSCA: Listed (active)
- OSHA HazCom 2012: Fully compliant GHS alignment required
- DOT Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S. (Ethyl methanesulfonate)
- Hazard Class: 6.1 (Toxic substances)
- Packing Group: II
- Label: Skull and crossbones, health hazard
- EPA Reportable Quantity (RQ): 1 lb (0.45 kg) – CERCLA reportable
European Union (REACH, CLP, ADR)
- REACH Registered: Yes (Registration required under Title II)
- SVHC Candidate List: Not currently listed, but mutagenicity may trigger future review
- CLP Regulation: Fully aligned with GHS classifications above
- ADR (Road Transport): Class 6.1, UN 2924, PG II
Canada (WHMIS, TDG)
- WHMIS 2015: Fully classified under GHS
- TDG (Transportation of Dangerous Goods): Class 6.1, UN 2924, Packing Group II
China (MEP, GHS)
- Listed under China GHS (5th Edition)
- Requires hazardous chemical registration under Measures for the Administration of Hazardous Chemicals
India (Manufacture, Storage, Import of Hazardous Chemicals Rules)
- Requires prior authorization for import and handling
- Subject to Hazardous Waste Management Rules if discarded
4. Storage & Handling
- Storage Conditions:
- Store in a cool, dry, well-ventilated area
- Keep away from heat, sparks, open flames
- Store in original, tightly sealed container
- Isolate from oxidizers, bases, and foodstuffs
-
Use only in a fume hood with local exhaust ventilation
-
Handling Precautions:
- Use only with appropriate PPE (nitrile gloves, lab coat, safety goggles, face shield)
- Avoid skin and eye contact; do not inhale vapors
- Ground containers during transfer to prevent static discharge
5. Packaging & Transport (IATA/IMDG/ADR)
- Proper Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S. (Ethyl methanesulfonate)
- UN Number: UN 2924
- Class: 6.1 (Toxic substances)
- Packing Group: II
- IATA (Air): PI 602 or PI 650 (for limited quantities)
- IMDG (Sea): IMDG Code, Special Provision 274, stowage category C
- ADR (Road, Europe): Tunnel Code C
Note: Limited and excepted quantities may apply for small shipments under specific conditions.
6. Environmental & Disposal Considerations
- Environmental Hazard: Toxic to aquatic organisms; do not release into environment
- Waste Disposal:
- Follow local, national, and international regulations (e.g., RCRA in the US, Basel Convention)
- Dispose as hazardous waste through licensed facility
- Incineration in approved hazardous waste incinerator with scrubbing
7. Emergency Response
- Spill Response:
- Evacuate area, ventilate
- Wear full PPE (respirator, chemical suit)
- Contain spill with inert absorbent (vermiculite, sand); place in sealed container
-
Decontaminate area with appropriate neutralizer (consult SDS)
-
Fire Hazards:
- Not flammable but may emit toxic fumes (SOx, CO) when heated
-
Use water spray, alcohol-resistant foam, dry chemical
-
First Aid:
- Inhalation: Move to fresh air; administer oxygen if needed; seek medical attention
- Skin Contact: Remove contaminated clothing; flush with water for 15+ minutes; seek medical help
- Eye Contact: Flush with water for 15+ minutes; consult ophthalmologist
- Ingestion: Rinse mouth; do NOT induce vomiting; seek immediate medical assistance
8. Documentation Requirements
- Safety Data Sheet (SDS): Required (ISO 11014 / GHS-compliant)
- Transport Documents: Include UN number, proper shipping name, class, packing group, and emergency contact
- Import/Export Permits: May be required depending on country (e.g., APHIS, DGFT in India, REACH authorization review if applicable)
- Customs Declaration: HS Code 2904.90 must be declared accurately
9. Key Compliance Tips
- Always verify HS code 2904.90 with national customs authority (some countries may subclassify)
- Monitor regulatory updates due to mutagenicity classification
- Train personnel on mutagen handling protocols (especially in labs)
- Use dedicated equipment to prevent cross-contamination
Disclaimer: This guide is for informational purposes only. Regulations vary by jurisdiction. Always consult current SDS, local regulatory bodies, and legal counsel before transporting, storing, or using Ethyl Methanesulfonate.
In conclusion, sourcing ethyl methanesulfonate requires careful consideration of safety, regulatory compliance, and supplier reliability. As a potent alkylating agent with potential mutagenic and toxic properties, it is essential to obtain this chemical from reputable suppliers who adhere to strict quality control and safety standards. Proper documentation, including safety data sheets (SDS), should be reviewed to ensure safe handling, storage, and disposal. Researchers and industrial users must comply with local regulations regarding hazardous chemicals, including obtaining necessary permits or approvals where required. Additionally, evaluating alternatives or safer analogs may be prudent depending on the application. Ultimately, responsible sourcing of ethyl methanesulfonate involves balancing scientific needs with ethical, environmental, and occupational safety responsibilities.