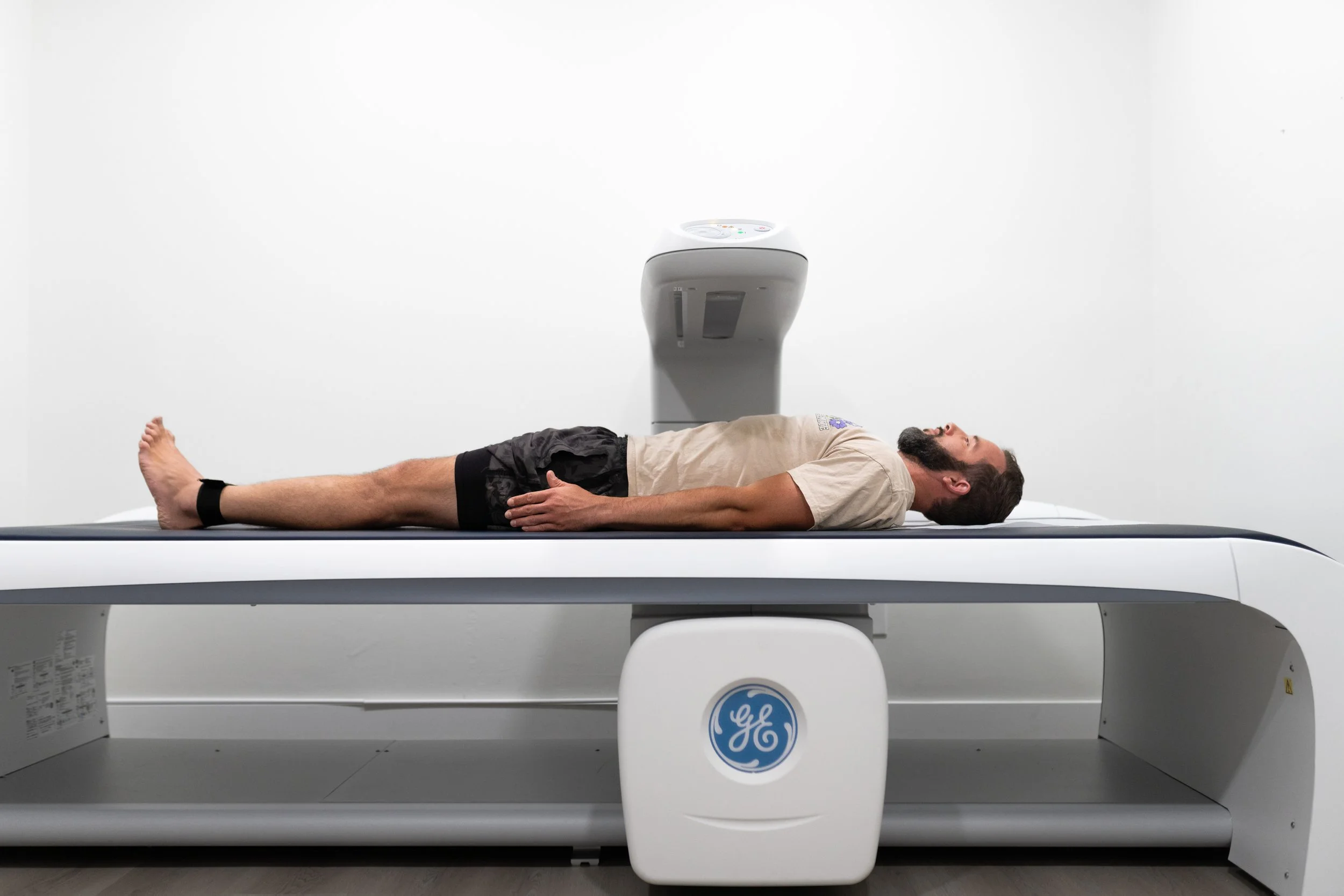
The global DEXA (Dual-Energy X-ray Absorptiometry) scan equipment market is experiencing robust growth, driven by rising prevalence of osteoporosis, increasing geriatric population, and growing awareness about early diagnosis of bone disorders. According to Grand View Research, the global bone densitometry market size was valued at USD 1.2 billion in 2023 and is projected to expand at a compound annual growth rate (CAGR) of 6.8% from 2024 to 2030. This expanding demand has intensified competition among medical device manufacturers, fostering innovation in accuracy, speed, and patient accessibility across DEXA technologies. As healthcare facilities seek reliable, high-performance systems, a handful of key players have emerged as leaders in producing advanced DEXA scan equipment. Based on market presence, technological innovation, and clinical adoption rates, the following are the top 10 DEXA scan equipment manufacturers shaping the future of bone health diagnostics.
Top 10 Dexa Scan Equipment Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Fitnescity DEXA Body Composition Scan
Domain Est. 1998
Website: questhealth.com
Key Highlights: Free deliveryThe DEXA Scan provides a detailed, segmental analysis of your fat and lean mass, visceral fat, and bone density measurements.Missing: equipment manufacturer…
#2 BeamMed Bone Density
Domain Est. 2005
Website: beammed.com
Key Highlights: BeamMed is a leader in the market for advanced ultrasound technology & portable bone density devices that allow physicians early assessment of osteoporosis….
#3 Get DexaFit
Domain Est. 2011
Website: dexafit.com
Key Highlights: Unlock precise health insights with DexaFit’s AI-enhanced DEXA scans, the gold standard for accurate body composition and skeletal health assessments.Missing: equipment manufactur…
#4 DEXA (DXA) Scan Machines
Domain Est. 1995
Website: henryschein.com
Key Highlights: Shop from a selection of our featured DEXA (DXA) Scan Machines and Bone Density Scanners. Fill out the form to contact a sales rep and get started!…
#5 Hologic
Domain Est. 1995
Website: hologic.com
Key Highlights: Hologic helps women around the world live healthier lives. We develop innovative medical technologies that effectively detect, diagnose and treat health ……
#6 Bone and Metabolic Health
Domain Est. 1999
Website: gehealthcare.com
Key Highlights: Lunar DXA systems lead the way in bone and metabolic health innovation, empowering customers with trusted clinical solutions that enhance patient outcomes….
#7
Domain Est. 2009
Website: dexasolutions.com
Key Highlights: DEXA Solutions, LLC is the only business in that world that exclusively provides bone density machine support for a wide range of industries….
#8 BodySpec: Full
Domain Est. 2011
Website: bodyspec.com
Key Highlights: BodySpec DEXA scans give precise body fat, muscle, and bone density metrics in 15 minutes, empowering smarter training, nutrition, and health decisions….
#9
Domain Est. 2016
Website: dms-imaging.com
Key Highlights: DMS Imaging is focused on the development, design and manufacturing of medical imaging systems mainly for digital radiology and bone densitometry….
#10 DEXA Plus
Domain Est. 2018
Website: dexaplus.com
Key Highlights: Discover how DEXA Plus helps healthcare providers, wellness centers, and sports teams grow revenue with state-of-the-art DEXA scanners….
Expert Sourcing Insights for Dexa Scan Equipment

H2: 2026 Market Trends for DEXA Scan Equipment
The global market for Dual-Energy X-ray Absorptiometry (DEXA or DXA) scan equipment is poised for significant transformation by 2026, driven by demographic shifts, technological innovation, and increasing awareness of musculoskeletal and metabolic health. As osteoporosis, sarcopenia, and obesity continue to rise worldwide, DEXA technology remains a gold standard for body composition and bone mineral density (BMD) assessment. Key trends shaping the DEXA equipment market in 2026 include:
-
Growing Prevalence of Osteoporosis and Chronic Diseases
With aging populations in North America, Europe, and parts of Asia-Pacific, the incidence of osteoporosis and fracture-related morbidity is escalating. The World Health Organization estimates that over 200 million people globally suffer from osteoporosis, intensifying demand for early diagnosis. DEXA scans are critical in risk assessment, fueling adoption in primary care, endocrinology, and geriatric clinics. -
Expansion into Obesity and Metabolic Health Monitoring
Beyond bone health, DEXA’s ability to precisely measure fat mass, lean muscle, and visceral adiposity is expanding its use in obesity management, sports medicine, and bariatric surgery follow-up. By 2026, clinics specializing in metabolic health and wellness are expected to integrate DEXA as a standard tool, increasing market penetration in non-traditional healthcare settings. -
Technological Advancements and AI Integration
Next-generation DEXA systems are incorporating artificial intelligence (AI) and machine learning algorithms to enhance image analysis, reduce scan time, and improve diagnostic accuracy. Automated reporting, cloud-based data storage, and predictive analytics for fracture risk are becoming standard features. Vendors are also introducing portable and low-dose DEXA devices, broadening access in outpatient and rural clinics. -
Increased Demand in Emerging Markets
Countries in Latin America, Southeast Asia, and the Middle East are witnessing rising healthcare investments and improved diagnostic infrastructure. Government initiatives to combat non-communicable diseases and growing insurance coverage are enabling wider adoption of DEXA technology in private hospitals and diagnostic centers. -
Regulatory and Reimbursement Landscape
Favorable reimbursement policies in the U.S. (e.g., Medicare coverage for BMD testing) continue to support market growth. However, inconsistent reimbursement in some regions remains a barrier. By 2026, advocacy groups and manufacturers are expected to push for standardized guidelines and expanded insurance coverage, especially for body composition analysis in chronic disease management. -
Competitive Landscape and Strategic Alliances
Major players such as Hologic, GE HealthCare (now part of General Electric), and Lunar (a subsidiary of GE) dominate the market. Intense competition is driving innovation and partnerships with digital health platforms. Smaller companies are entering with niche, cost-effective DEXA solutions tailored for fitness centers and research institutions. -
Home and Point-of-Care Applications
Although still in early stages, the development of compact, hand-held, or mobile DEXA units could enable point-of-care testing in pharmacies, sports facilities, and even home health settings by the late 2020s. While regulatory and accuracy challenges remain, pilot programs in 2025–2026 may pave the way for decentralized bone and body composition screening.
In summary, the DEXA scan equipment market in 2026 is characterized by diversification in clinical applications, technological sophistication, and geographic expansion. As healthcare systems prioritize preventive care and personalized medicine, DEXA technology is expected to transition from a specialized diagnostic tool to a broader health monitoring asset, supporting long-term market growth.

Common Pitfalls When Sourcing DEXA Scan Equipment: Quality and Intellectual Property Risks
Sourcing DEXA (Dual-Energy X-ray Absorptiometry) scan equipment involves significant technical, regulatory, and financial considerations. Buyers must be vigilant to avoid common pitfalls related to equipment quality and intellectual property (IP) infringement, which can lead to operational failures, legal liabilities, and compromised patient care.
Poor Equipment Quality and Performance
One of the most significant risks when sourcing DEXA equipment—especially from non-OEM (Original Equipment Manufacturer) or secondary markets—is acquiring systems that do not meet clinical or regulatory standards. Low-quality or refurbished units may suffer from outdated software, hardware degradation, or inaccurate calibration, leading to unreliable bone density measurements. Poor image quality or inconsistent scan results can compromise diagnostic accuracy and expose healthcare providers to clinical and legal risks. Additionally, equipment lacking current regulatory certifications (such as FDA 510(k) clearance or CE marking) may not be legally deployable in certain regions.
Inadequate or Missing Service and Support
Many third-party suppliers fail to provide comprehensive technical support, maintenance contracts, or access to genuine spare parts. DEXA systems require regular calibration, software updates, and trained service engineers to ensure proper function. Sourcing from vendors without established service networks can result in prolonged downtime, increased repair costs, and voided warranties. Buyers may also encounter difficulties in obtaining compatible software upgrades, further reducing equipment longevity and clinical utility.
Intellectual Property Infringement Risks
Purchasing DEXA equipment from unauthorized or gray-market suppliers increases the risk of IP violations. Some vendors may offer systems with pirated or illegally cloned software, modified firmware, or counterfeit components. Using such equipment can expose the buyer to legal action from the original manufacturer for copyright or patent infringement. Additionally, tampered software may lack critical security updates or fail to comply with data protection regulations like HIPAA or GDPR, leading to potential data breaches and compliance penalties.
Lack of Regulatory Compliance and Traceability
Equipment sourced through unofficial channels may lack proper documentation, including certificates of conformity, service history, or proof of origin. This absence of traceability raises concerns about whether the device meets current safety and performance standards. Regulatory bodies may deem non-compliant systems unfit for clinical use, resulting in costly retrofits or forced decommissioning. Moreover, institutions may struggle to validate equipment for accreditation purposes without verifiable compliance records.
Hidden Costs and Total Cost of Ownership
While initial purchase prices from third-party suppliers may appear attractive, hidden costs often emerge post-acquisition. These include fees for software licensing reinstatement, mandatory upgrades, compliance validation, and higher maintenance expenses due to lack of OEM support. Buyers may also face unexpected costs related to IP disputes or regulatory fines, significantly increasing the total cost of ownership over the equipment’s lifecycle.
Conclusion
To mitigate these risks, buyers should prioritize sourcing DEXA scan equipment through authorized distributors or directly from OEMs. Conducting thorough due diligence—including verification of regulatory status, software licensing, service agreements, and IP legitimacy—is essential. Investing in quality and compliance upfront ensures reliable clinical performance, legal protection, and long-term operational efficiency.

Logistics & Compliance Guide for DEXA Scan Equipment
Overview
Dual-Energy X-ray Absorptiometry (DEXA or DXA) scan equipment is used to measure bone mineral density and body composition. Due to the integration of X-ray technology, its transportation, installation, and operation are subject to strict regulatory and compliance requirements. This guide outlines key logistics and compliance considerations for handling DEXA equipment from procurement to clinical use.
Regulatory Classification
DEXA scan devices are classified as medical devices and often as radiation-emitting electronic products. In the United States, they are regulated by the Food and Drug Administration (FDA) under 21 CFR Part 1020.30. Internationally, compliance with the International Electrotechnical Commission (IEC) standards such as IEC 60601-1 (safety) and IEC 60601-2-54 (particular requirements for X-ray equipment) is required.
Transportation & Shipping
- Packaging: DEXA equipment must be shipped in manufacturer-approved, shock-resistant packaging with secure immobilization of sensitive components (e.g., X-ray tube, detector).
- Hazard Labeling: Packages containing X-ray components must be labeled in accordance with IATA/ICAO regulations if air-freighted, including “Radioactive Material” exemptions (note: DEXA equipment is typically non-radioactive but may require special handling labels due to X-ray generation).
- Carrier Requirements: Use freight carriers experienced in handling medical imaging equipment. Temperature and humidity controls may be required during transit.
- Documentation: Include packing lists, safety data sheets (if applicable), import permits, and regulatory compliance certificates (e.g., CE mark, FDA 510(k) clearance).
Import/Export Compliance
- Customs Documentation: Prepare commercial invoices, certificates of origin, and FDA Form 2877 (Notice of Arrival) for U.S. imports.
- Dual-Use Considerations: DEXA systems may be subject to export control regulations (e.g., EAR – Export Administration Regulations) due to advanced imaging technology. Verify Export Control Classification Number (ECCN).
- Country-Specific Requirements: Some countries require pre-approval from health or radiation safety authorities (e.g., Health Canada, MHRA in the UK, TGA in Australia).
Installation & Site Preparation
- Facility Requirements:
- Radiation shielding (e.g., lead-lined walls) if required by local regulations.
- Stable power supply with surge protection (refer to manufacturer specifications).
- Adequate space with proper ventilation and environmental controls (temperature: 10–35°C, humidity: 30–75%).
- Radiation Safety Assessment: Conduct a site evaluation by a qualified medical physicist or radiation safety officer (RSO) before installation.
- Regulatory Notification: In many jurisdictions, facilities must notify or register the installation of X-ray equipment with the state or national radiation protection authority (e.g., state health department in the U.S.).
Regulatory Registration & Licensing
- Equipment Registration: Register the DEXA unit with the appropriate regulatory body (e.g., state radiation control program in the U.S.).
- Facility License: Ensure the healthcare facility holds a valid license to operate radiation-producing equipment.
- Operator Certification: Personnel operating DEXA equipment must be appropriately trained and certified (e.g., ARRT certification in radiography or specialized DEXA certification like ISCD).
Quality Assurance & Maintenance
- Routine Testing: Perform daily, monthly, and annual quality control (QC) tests as per manufacturer and professional society guidelines (e.g., ISCD, ISMRM).
- Calibration: Equipment must be calibrated annually by a qualified service engineer or medical physicist.
- Service Records: Maintain logs of maintenance, repairs, and QC results for regulatory audits.
Data Security & HIPAA Compliance (U.S.)
- Patient Data Protection: DEXA systems store personal health information (PHI). Ensure data is encrypted, access is role-based, and audit trails are enabled.
- HIPAA Compliance: Follow HIPAA Security Rule requirements for electronic protected health information (ePHI), including secure network configurations and regular risk assessments.
Decommissioning & Disposal
- Radiation Safety Survey: Conduct a final radiation survey before decommissioning.
- Data Wipe: Securely erase all patient data from internal systems and storage devices.
- E-Waste Compliance: Dispose of electronic components in accordance with local e-waste regulations (e.g., WEEE Directive in the EU).
- X-ray Tube Handling: Follow manufacturer and environmental guidelines for disposal of the X-ray tube (may contain hazardous materials).
Training & Documentation
- Staff Training: Provide documented training on safe operation, emergency procedures, and radiation safety.
- Compliance Files: Maintain a compliance binder including:
- Equipment registration
- Operator certifications
- QC logs
- Service records
- Radiation safety program documents
Conclusion
Proper logistics planning and strict adherence to compliance requirements are essential for the safe and legal deployment of DEXA scan equipment. Facilities must coordinate with regulatory bodies, qualified professionals, and manufacturers throughout the equipment lifecycle to ensure patient safety, data integrity, and regulatory compliance.
Conclusion for Sourcing DEXA Scan Equipment:
After a comprehensive evaluation of available DEXA (Dual-Energy X-ray Absorptiometry) scan equipment options, it is clear that selecting the right system involves balancing clinical needs, budget constraints, facility requirements, and future scalability. Key factors such as image accuracy, software capabilities, patient throughput, regulatory compliance (e.g., FDA clearance), vendor support, and training must be carefully weighed.
The market offers a range of systems—from full-body scanners for comprehensive bone density and body composition analysis to peripheral models suited for focused screening. Established manufacturers like Hologic, GE Healthcare, and Lunar (a GE subsidiary) continue to lead in reliability and technological innovation, while emerging brands offer competitive pricing and modern features that may suit smaller or budget-conscious practices.
Ultimately, the optimal choice depends on the intended use (e.g., osteoporosis diagnosis, research, sports medicine, or weight management), patient volume, available space, and long-term operational goals. Prioritizing strong vendor partnerships with robust service and maintenance plans will ensure equipment longevity and minimize downtime.
In conclusion, a strategic sourcing approach—guided by clinical objectives, total cost of ownership, and future growth—will enable healthcare providers to acquire DEXA equipment that enhances diagnostic accuracy, improves patient care, and delivers a strong return on investment.