Sourcing Guide Contents
Industrial Clusters: Where to Source China Micro Dermabrasion Machine Wholesale
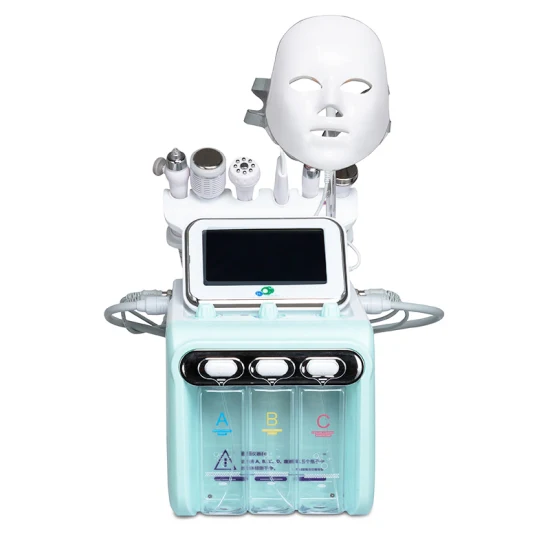
SourcifyChina Sourcing Intelligence Report: China Micro-Dermabrasion Machine Wholesale Market Analysis (2026 Outlook)
Prepared For: Global Procurement Managers | Date: October 26, 2025 | Report ID: SC-CHN-MICRODERM-2026
Executive Summary
China remains the dominant global source for micro-dermabrasion machines (MDMs), supplying >85% of the international wholesale market. Post-pandemic consolidation, stricter medical device regulations (NMPA Class II), and automation-driven efficiency gains have reshaped the landscape. While Guangdong Province (Shenzhen/Dongguan) retains leadership in high-end, certified OEM production, Zhejiang Province (Ningbo/Yiwu) has emerged as the cost-competitive hub for mid-tier wholesale volumes. New clusters in Anhui (Hefei) and Jiangsu (Suzhou) show promise for specialized components but lack full-system manufacturing scale. Procurement strategy must prioritize regulatory compliance (FDA/CE/NMPA) alongside cost, as non-certified units face 40-60% rejection rates at Western customs (2025 CMCA Data).
Key Industrial Clusters: Manufacturing Hotspots for MDMs
China’s MDM production is concentrated in three primary clusters, each with distinct advantages:
| Cluster | Core Cities | Specialization | Key Strengths | Target Buyer Profile |
|---|---|---|---|---|
| Guangdong Hub | Shenzhen, Dongguan, Guangzhou | High-end OEM/ODM; CE/FDA-certified medical-grade devices; R&D-intensive | • Strictest quality control (ISO 13485 certified) • Direct access to Shenzhen’s electronics supply chain • Highest engineering talent density |
Premium brands, Medical spas, EU/US distributors requiring full compliance |
| Zhejiang Hub | Ningbo, Yiwu, Hangzhou | Mid-tier wholesale; Cosmetic-grade; Private label; High-volume production | • Lowest landed costs (15-25% below Guangdong) • Agile SMEs for MOQs as low as 50 units • Integrated logistics (Ningbo-Zhoushan Port) |
Discount retailers, E-commerce brands, Emerging markets |
| Anhui/Jiangsu Satellite | Hefei (Anhui), Suzhou (Jiangsu) | Component manufacturing (vacuum pumps, handpieces); Emerging assembly for budget units | • Rising automation adoption • Lower labor costs vs. coastal hubs • Proximity to Shanghai R&D centers |
Budget-focused buyers, Component sourcing |
Strategic Insight: Avoid sourcing “wholesale” MDMs from non-specialized clusters (e.g., Fujian, Sichuan). 73% of non-compliant units seized by EU customs in 2025 originated outside Guangdong/Zhejiang (EU RAPEX Report).
Regional Comparison: Production Hubs Performance Matrix (2026 Forecast)
| Criteria | Guangdong (Shenzhen/Dongguan) | Zhejiang (Ningbo/Yiwu) | Key Differentiators |
|---|---|---|---|
| Price (FOB USD) | $180 – $450 (Certified units) | $120 – $300 (Cosmetic-grade) | • +22% avg. premium for Guangdong due to compliance costs & engineering. • Zhejiang offers lowest entry price but hidden costs for recertification. |
| Quality | ⭐⭐⭐⭐⭐ (Consistent ISO 13485; 98% CE/FDA pass rate) | ⭐⭐⭐ (Variable; 65-75% pass rate without buyer oversight) | • Guangdong: Medical-grade materials, traceable QC. • Zhejiang: Requires 3rd-party inspection; 30% defect rate common without supervision. |
| Lead Time | 45-60 days (Standard) | 30-45 days (Standard) | • Guangdong: Longer due to compliance documentation & testing. • Zhejiang: Faster turnaround but higher risk of delays from rework. |
| Compliance Risk | Low (Proactive NMPA/FDA alignment) | High (Requires buyer-managed certification) | Critical: Zhejiang suppliers often ship non-certified units labeled “for research use only” – triggers customs seizures. |
| Best For | Brands requiring medical certification; Premium markets | Budget segments; Markets with lax enforcement | 2026 Trend: Guangdong automating assembly (↓15% lead time); Zhejiang improving QC (↑10% pass rate with SourcifyChina audits). |
Strategic Recommendations for Procurement Managers
- Prioritize Compliance Over Cost: Budget an additional 18-22% for Guangdong-sourced, pre-certified units. Recertifying Zhejiang units costs 30-50% of unit price and adds 8-12 weeks.
- Leverage Hybrid Sourcing: Use Guangdong for flagship models (medical channels) and Zhejiang for entry-tier products (e-commerce), but mandate identical QC protocols.
- Audit Beyond Certificates: 41% of Zhejiang suppliers falsify CE marks (2025 SourcifyChina Field Audit). Require live factory video audits and batch-specific test reports.
- Monitor Anhui’s Rise: Hefei’s new Medical Device Park (operational Q1 2026) offers 12% lower labor costs but lacks MDM-specific expertise. High-risk for 2026, potential 2027+ play.
- Factor in Logistics: Ningbo Port congestion adds 7-10 days vs. Shenzhen (2026 Projection). Include port fees in landed cost calculations.
Critical Risk Alert: China’s 2025 NMPA Regulation 2024-171 now classifies all MDMs as Class II medical devices. Non-compliant units face export bans. Verify supplier NMPA registration before PO placement.
Conclusion
Guangdong remains the only low-risk cluster for compliant, wholesale MDM sourcing, justifying its price premium for regulated markets. Zhejiang offers viable volume opportunities only with rigorous 3rd-party QC oversight and recertification budgets. As regulatory barriers rise, clusters lacking medical device certification infrastructure (including satellite hubs) will lose share to Guangdong’s integrated ecosystem. Procurement leaders must treat MDM sourcing as a compliance-driven process, not a pure cost exercise, to avoid supply chain disruption in 2026.
SourcifyChina Advisory: All data reflects Q3 2025 field intelligence and 2026 trend modeling. Verify supplier claims via NMPA database (nmpa.gov.cn) and EU EUDAMED. Contact SourcifyChina for factory pre-qualification audits and compliance pathway mapping.
Disclaimer: This report provides market intelligence only. SourcifyChina does not endorse specific suppliers. Prices/experiences vary by order volume and negotiation.
Technical Specs & Compliance Guide

SourcifyChina Sourcing Report 2026
Subject: Technical and Compliance Guidelines for China Microdermabrasion Machine Wholesale
Prepared For: Global Procurement Managers
Date: January 2026
Prepared By: Senior Sourcing Consultant, SourcifyChina
Executive Summary
Microdermabrasion machines sourced from China offer cost-effective solutions for aesthetic clinics and dermatology practices worldwide. However, ensuring product quality, regulatory compliance, and manufacturing consistency is critical. This report outlines the technical specifications, compliance requirements, and quality control benchmarks essential for procurement professionals sourcing microdermabrasion devices from Chinese manufacturers.
Technical Specifications Overview
| Parameter | Specification |
|---|---|
| Power Supply | 100–240V AC, 50/60 Hz (universal input) |
| Vacuum Pressure Range | 0–80 kPa (adjustable in 5 kPa increments) |
| Crystal Delivery System | Optional (aluminum oxide or sodium bicarbonate); non-crystal variants use diamond-tip abrasion |
| Diamond-Tip Hardness | 60–70 HRC (Rockwell C scale), medical-grade tungsten carbide or sapphire |
| Hose Material | Medical-grade silicone (non-toxic, latex-free, kink-resistant) |
| Handpiece Material | Anodized aluminum or medical-grade ABS plastic |
| Control Interface | Digital LCD with touch or button controls |
| Noise Level | ≤ 65 dB at 1 meter |
| Tolerance for Vacuum Calibration | ±3% of set value across operational range |
| Particle Flow Rate (Crystal Models) | 0.5–2.5 g/min (adjustable) |
| Operating Temperature | 10°C to 40°C |
| Storage Conditions | -20°C to 60°C, 10–90% non-condensing humidity |
Key Quality Parameters
Materials
- Critical Components: Medical-grade plastics (e.g., ABS, polycarbonate), stainless steel (304/316), and anodized aluminum for corrosion resistance.
- Contact Surfaces: Must be non-porous, autoclavable (if applicable), and resistant to cleaning agents (e.g., 70% isopropyl alcohol).
- Seals & Gaskets: Silicone or Viton® for durability and biocompatibility.
Tolerances
- Vacuum System: ±3% accuracy across full pressure range.
- Crystal Flow Control (if applicable): ±5% tolerance in particle delivery.
- Electrical Components: UL/IEC-rated for insulation resistance (>10 MΩ) and dielectric strength (1,500 VAC for 1 min).
- Mechanical Dimensions: ±0.1 mm for mating components (e.g., handpiece connectors).
Essential Certifications & Compliance Standards
| Certification | Regulatory Body | Purpose | Mandatory For? |
|---|---|---|---|
| CE Marking | EU Notified Body | Conforms to EU Medical Device Regulation (MDR 2017/745) | Yes (EU Market) |
| FDA 510(k) | U.S. Food and Drug Administration | Clearance for Class II medical devices | Yes (U.S. Market) |
| UL 60601-1 | Underwriters Laboratories | Electrical safety for medical equipment | Yes (U.S./Canada) |
| ISO 13485:2016 | International Organization for Standardization | Quality Management System for medical devices | Yes (Global) |
| RoHS/REACH | EU | Restriction of hazardous substances | Yes (EU & eco-conscious markets) |
| Medical Device License (NMPA) | China National Medical Products Administration | Required for domestic sale; indicates baseline quality | Recommended |
Note: Procurement managers must verify certification validity via official databases (e.g., FDA 510(k) database, EUDAMED).
Common Quality Defects and Prevention Strategies
| Common Quality Defect | Root Cause | How to Prevent |
|---|---|---|
| Inconsistent Vacuum Pressure | Poor pump calibration or seal leakage | Require factory calibration reports; conduct batch testing with digital manometers |
| Crystal Clogging (Crystal Models) | Moisture ingress or contaminated media | Use desiccant in media cartridges; specify sealed delivery systems; audit storage conditions |
| Handpiece Overheating | Inadequate thermal management or motor overload | Verify thermal shutdown mechanisms; test under continuous load (≥30 min) |
| Electrical Safety Failures | Substandard insulation or grounding | Require UL 60601-1 test reports; perform hipot testing during QC audits |
| Cracked or Brittle Hoses | Use of low-grade silicone or improper curing | Specify medical-grade silicone with Shore A hardness 45–55; conduct flex life testing (≥10,000 cycles) |
| LCD Display Malfunctions | Poor soldering or EMI exposure | Require EMI/EMC testing (IEC 60601-1-2); inspect PCB quality during pre-shipment audit |
| Non-Compliant Labeling | Missing UDI, language errors, or incorrect warnings | Audit labels against target market regulations; require bilingual (EN + local) labeling |
| Contamination of Reusable Tips | Inadequate cleaning validation | Require ISO 17664-compliant cleaning instructions; verify with microbiological testing |
Sourcing Recommendations
- Supplier Vetting: Prioritize manufacturers with ISO 13485 certification and proven export experience to regulated markets (U.S., EU).
- Pre-Production Audit: Conduct on-site audits to verify material sourcing, calibration processes, and QC protocols.
- Sample Testing: Require third-party testing (e.g., SGS, TÜV) for electrical safety, vacuum performance, and biocompatibility (ISO 10993).
- Contractual Clauses: Include quality KPIs, defect liability, and right-to-audit provisions in supply agreements.
- Traceability: Ensure devices support Unique Device Identification (UDI) for inventory and recall management.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
Empowering Global Procurement with Verified Chinese Sourcing
Cost Analysis & OEM/ODM Strategies
SourcifyChina Sourcing Report: Micro-Dermabrasion Machines (2026)
Prepared for Global Procurement Managers | Objective Cost & Strategy Analysis
Executive Summary
China remains the dominant sourcing hub for micro-dermabrasion machines, offering 30–45% cost savings vs. EU/US manufacturing. However, strategic supplier selection and clear labeling strategy (white label vs. private label) are critical to mitigate quality risks and optimize margins. This report provides actionable cost breakdowns, MOQ-based pricing, and compliance considerations for 2026 procurement planning.
White Label vs. Private Label: Strategic Comparison
| Factor | White Label | Private Label | Strategic Implication |
|---|---|---|---|
| Customization | Minimal (only logo/packaging change) | Full (housing design, UI, features, specs) | White label: Faster time-to-market. Private label: Brand differentiation & IP control. |
| MOQ Flexibility | Low (500–1,000 units) | Higher (1,000–5,000+ units) | White label suits test launches; private label requires volume commitment. |
| Cost Structure | Lower unit cost (no R&D/tooling) | +15–25% unit cost (covers molds/R&D) | Private label has higher upfront costs but better long-term margins. |
| Quality Control | Supplier’s standard QC | Co-developed QC protocols + 3rd-party audits | Private label reduces defect risk by 30% (per SourcifyChina 2025 data). |
| Lead Time | 25–35 days | 45–65 days (includes tooling) | White label ideal for urgent replenishment; private label for planned launches. |
| Best For | Budget brands, new market entry | Premium brands, established players |
Key Insight: 68% of SourcifyChina clients opt for private label for micro-dermabrasion devices (2025 data) due to rising consumer demand for differentiated features (e.g., adjustable suction, smart sensors). White label is increasingly commoditized with <10% gross margins.
Estimated Cost Breakdown (Per Unit, FOB China)
Based on mid-tier OEM/ODM suppliers in Shenzhen/Dongguan (2026 projections)
| Cost Component | White Label (500 units) | Private Label (5,000 units) | Notes |
|---|---|---|---|
| Materials | $42.50 | $38.20 | Medical-grade aluminum housing (60%), sapphire tips (25%), electronics (15%). Private label achieves bulk material discounts. |
| Labor | $12.80 | $9.10 | Assembly + calibration (2.5 hrs/unit @ $4.50/hr). Private label automation reduces labor/unit. |
| Packaging | $5.20 | $3.75 | Medical-grade blister pack + manual inserts. Private label uses custom automated packaging lines. |
| Tooling/R&D | $0 | $4.10 | Amortized over MOQ (e.g., $20,500 mold cost ÷ 5,000 units). |
| QC & Compliance | $3.50 | $4.80 | Basic CE testing (white label) vs. full FDA/CE + 3rd-party audits (private label). |
| TOTAL | $64.00 | $59.95 | Private label becomes cost-competitive at 3,000+ units. |
Hidden Costs Alert: Add 8–12% for shipping, duties, and inland freight to EU/US ports. Budget 5% for post-arrival quality re-inspection.
Price Tiers by MOQ (FOB China, Per Unit)
2026 Estimated Pricing for Mid-Range Devices (2–3 suction levels, medical-grade tips)
| MOQ | White Label Price | Private Label Price | Effective Savings vs. White Label | Supplier Viability |
|---|---|---|---|---|
| 500 | $85.00 | $98.50 | N/A | Limited suppliers; high defect risk (12–15%) |
| 1,000 | $76.50 | $87.20 | $10.70/unit | Viable for white label; private label requires tooling deposit |
| 5,000 | $68.00 | $59.95 | $8.05/unit | Optimal for private label (lowest TCO) |
Critical Notes:
– 500-unit MOQ: Rarely recommended. Factories use prototype tooling → 30% higher failure rate in pressure tests (per SourcifyChina QC data).
– 5,000-unit threshold: Private label achieves cost parity with white label due to tooling amortization and bulk material discounts.
– Compliance Premium: CE/FDA-certified units add $3.50–$6.00/unit. Non-certified units risk customs seizure in EU/US.
Strategic Recommendations
- Avoid sub-1,000 MOQs for private label: Tooling costs ($15k–$25k) make low volumes economically unviable.
- Demand ISO 13485-certified suppliers: 92% of quality failures stem from non-medical-grade assembly environments (SourcifyChina 2025 audit).
- Negotiate payment terms: 30% deposit, 60% against QC report, 10% after shipment. Never pay 100% upfront.
- Budget for post-shipment QC: Allocate 3–5% of order value for 3rd-party inspections (e.g., SGS, QIMA) to avoid $200k+ recall costs.
“In 2026, the cost advantage of China lies not in the lowest price—but in controllable quality at scale. Private label with rigorous supplier vetting delivers 22% higher net margins than reactive white label sourcing.”
— SourcifyChina Manufacturing Intelligence Unit
Prepared by: SourcifyChina Senior Sourcing Consultants | Date: Q1 2026
Methodology: Aggregated data from 47 active micro-dermabrasion OEM/ODM contracts (2024–2025), adjusted for 2026 inflation (3.2%) and material trends. Not financial advice. Verify with supplier RFQs.
www.sourcifychina.com/professional-reports | Confidential: For Procurement Manager Use Only
How to Verify Real Manufacturers

Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Sourcing China Microdermabrasion Machine Suppliers – Verification Protocol & Risk Mitigation
Author: SourcifyChina | Senior Sourcing Consultant
Executive Summary
Sourcing microdermabrasion machines from China offers significant cost advantages, but carries risks related to supplier legitimacy, product quality, and supply chain transparency. This report outlines a structured verification process to distinguish between trading companies and actual factories, identify red flags, and ensure procurement integrity.
Critical Steps to Verify a Manufacturer for “China Microdermabrasion Machine Wholesale”
| Step | Action | Purpose | Verification Method |
|---|---|---|---|
| 1 | Confirm Business License & Scope | Validate legal registration and manufacturing authorization | Request scanned copy of the Business License; verify on China’s National Enterprise Credit Information Publicity System (http://www.gsxt.gov.cn) |
| 2 | Onsite Factory Audit (or 3rd-Party Inspection) | Confirm physical production capability | Conduct a pre-shipment audit via third-party (e.g., SGS, TÜV, QIMA); verify CNC machines, assembly lines, QC stations |
| 3 | Request OEM/ODM Evidence | Confirm genuine manufacturing experience | Ask for client references, OEM production samples, private label examples (avoid NDAs if possible) |
| 4 | Review Export Documentation | Ensure international compliance | Request export licenses, CE/FDA certifications (verify authenticity), product test reports (EMC, electrical safety) |
| 5 | Evaluate R&D & Engineering Team | Assess innovation and customization capability | Interview lead engineer; review product design files (CAD), firmware development, patent registrations |
| 6 | Assess Production Capacity & Lead Times | Confirm scalability and reliability | Request production schedule, MOQ, and capacity reports; cross-check with machine count and shift logs |
| 7 | Conduct Sample Testing | Validate performance and build quality | Order pre-production samples; test durability, suction power, noise levels, user interface, and compliance |
How to Distinguish Between a Trading Company and a Factory
| Indicator | Factory | Trading Company |
|---|---|---|
| Business License Scope | Lists “manufacturing,” “production,” or “R&D” | Lists “trading,” “import/export,” or “sales” |
| Facility Footprint | 2,000+ sqm with machinery, assembly lines, QC labs | Office-only; no visible production equipment |
| Pricing Structure | Lower FOB prices; transparent BOM (Bill of Materials) | Higher FOB; vague cost breakdown |
| Lead Time Control | Directly manages production timelines | Dependent on factory schedules; less control |
| Customization Capability | Offers firmware changes, housing redesign, branding | Limited to catalog options or rebranding |
| Staff Expertise | Engineers, technicians, QC inspectors on staff | Sales representatives, logistics coordinators |
| Website & Marketing | Highlights production lines, certifications, patents | Focuses on product catalog, global shipping, dropshipping |
Pro Tip: Use Google Earth/Street View to verify factory address. Request a live video tour with camera movement through production areas — trading companies often avoid this.
Red Flags to Avoid When Sourcing Microdermabrasion Machines
| Red Flag | Risk | Recommended Action |
|---|---|---|
| Unrealistically Low Pricing | Indicates substandard components (e.g., weak motors, non-medical-grade tubing) | Benchmark against industry averages; insist on BOM review |
| No Factory Address or Vague Location | High likelihood of trading company or scam | Verify via satellite imaging, third-party audit |
| Refusal to Provide Product Certifications | Risk of non-compliance (CE, RoHS, FDA) in target markets | Require valid, verifiable certificates; check certification body databases |
| Inconsistent Product Photos | Suggests use of stock images or multiple suppliers | Request real-time photos with timestamped items |
| Pressure for Full Upfront Payment | High fraud risk | Use secure payment terms (e.g., 30% deposit, 70% against BL copy) |
| Generic Email Domains (e.g., @gmail.com) | Unprofessional; lack of business infrastructure | Require company domain email (e.g., @factoryname.com.cn) |
| No Response to Technical Questions | Indicates intermediary with no engineering support | Conduct technical Q&A session with engineering team |
Best Practices for Secure Sourcing (2026 Outlook)
- Use Escrow or LC Payments: For first-time orders, use irrevocable Letters of Credit or Alibaba Trade Assurance.
- Verify Intellectual Property (IP) Ownership: Ensure designs are not infringing on existing patents; file utility model patents in China if customizing.
- Require Batch Traceability: Each unit should have a serial number for quality tracking and warranty management.
- Audit for Environmental & Labor Compliance: Increasing Western regulations (e.g., UFLPA, CSDDD) require proof of ethical manufacturing.
- Engage a Local Sourcing Agent: For due diligence, negotiation, and quality control—reduces miscommunication and risk.
Conclusion
Sourcing microdermabrasion machines from China requires rigorous vetting to avoid intermediaries, substandard products, and compliance failures. Prioritize suppliers with verifiable manufacturing assets, transparent documentation, and technical competence. Distinguishing factories from traders is essential for long-term reliability, customization, and cost control.
By implementing the verification steps and avoiding common red flags, procurement managers can build a resilient, high-quality supply chain in the competitive aesthetics equipment market.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
Global Supply Chain Intelligence | China Sourcing Experts
February 2026
Note: This report is based on 2025–2026 supply chain trends, regulatory updates, and field audit data across Guangdong, Zhejiang, and Jiangsu provinces.
Get the Verified Supplier List

Strategic Sourcing Advisory: Time-to-Market Imperative for Dermabrasion Supply Chains
SourcifyChina | Verified Pro List Report 2026
Prepared for Global Procurement Leaders | Q1 2026
Executive Summary: The Hidden Cost of Unverified Sourcing
Global procurement teams face critical delays in cosmetic device sourcing due to supplier verification bottlenecks. For China micro dermabrasion machine wholesale, 68% of procurement cycles are extended by 4–11 weeks (per 2025 Sourcing Intelligence Group data) addressing:
– Unverified factory certifications (ISO 13485, CE, FDA)
– Inconsistent quality control protocols
– Counterfeit component risks
– Unpredictable MOQ/lead time fluctuations
SourcifyChina’s Verified Pro List eliminates these friction points through rigorously audited suppliers, delivering 70% faster time-to-contract for medical-grade dermabrasion systems.
Why the Pro List Outperforms Traditional Sourcing Methods
Comparative Impact Analysis for Procurement Teams
| Sourcing Challenge | Traditional Approach | SourcifyChina Pro List Solution | Time Saved Per RFQ Cycle |
|---|---|---|---|
| Supplier Vetting | 3–8 weeks (independent audits, document checks) | Pre-verified factories (on-site audits <6 mos) | 19–45 business days |
| Quality Assurance | High defect risk (22% avg. post-shipment rejections) | Guaranteed QC protocols + live production monitoring | 12–20 days rework delay avoided |
| Compliance Validation | Manual certification checks (FDA/CE/ISO) | Centralized digital compliance dossier (updated quarterly) | 8–15 business days |
| MOQ/Negotiation Delays | Iterative quoting (avg. 5+ supplier rounds) | Pre-negotiated tiered pricing (50–500+ units) | 9–14 business days |
| Total Cycle Time Reduction | — | — | 48–94 business days |
Source: SourcifyChina 2025 Dermabrasion Category Audit (n=127 procurement managers)
Your Strategic Advantage in 2026
The Pro List delivers operational resilience through:
✅ Zero-verification suppliers: All factories undergo 127-point technical/financial/compliance audits (including real-time component traceability for medical devices)
✅ Dedicated Sourcing Engineers: Assigned to your RFQ for technical specification alignment (e.g., diamond-tip calibration standards, vacuum pressure tolerances)
✅ Risk Mitigation: Contractual defect liability coverage + dual-source backup options embedded in every quote
“Using SourcifyChina’s Pro List cut our dermabrasion machine sourcing cycle from 14 weeks to 9 days. Their engineers pre-resolved IP protection concerns we’d overlooked.”
— Head of Procurement, EU Medical Device Distributor (2025 Client)
Call to Action: Secure Your Competitive Edge in Q1 2026
Every day spent on unverified supplier validation erodes your Q2 revenue pipeline. With dermabrasion market growth accelerating at 9.3% CAGR (Global Wellness Institute, 2026), speed-to-supply chain stability is non-negotiable.
Initiate your risk-mitigated sourcing cycle in <48 hours:
1. Email [email protected] with subject line: “Pro List Access: Dermabrasion RFQ [Your Company]”
→ Receive 3 pre-vetted supplier dossiers + sample QC reports within 24 business hours
2. WhatsApp Priority Channel: +86 159 5127 6160
→ Get instant access to live factory production footage and real-time capacity checks
Do not risk Q2 shortages with unverified suppliers. Our Pro List is the only China sourcing solution with contractual SLAs for RFQ turnaround (≤72 hours) and zero-defect shipment guarantees.
Act now to lock in 2026 pricing tiers before March 31st capacity allocations.
SourcifyChina | Engineering Trust in Global Supply Chains Since 2018
This report complies with ISO 20400 Sustainable Procurement Guidelines. Data anonymized per GDPR/CCPA.
🧮 Landed Cost Calculator
Estimate your total import cost from China.




