Sourcing Guide Contents
Industrial Clusters: Where to Source China Implant Dental Lamp Wholesale
SourcifyChina Sourcing Intelligence Report 2026
Deep-Dive Market Analysis: Sourcing “China Implant Dental Lamp” Wholesale from China
Prepared for Global Procurement Managers | Q2 2026
Executive Summary
The global demand for dental implantation equipment, including specialized lighting systems—commonly referred to as implant dental lamps—has seen steady growth due to rising dental tourism, an aging population, and increased adoption of minimally invasive procedures. China has emerged as a dominant manufacturing hub for these precision medical devices, offering cost-competitive, high-performance solutions for wholesale procurement.
This report provides a strategic analysis of China’s manufacturing landscape for implant dental lamps, focusing on key industrial clusters, regional production strengths, and comparative sourcing metrics. The analysis is based on verified supplier data, factory audits, and trade flow intelligence collected through SourcifyChina’s on-the-ground network in 2025–2026.
Product Overview: China Implant Dental Lamp (Wholesale)
An implant dental lamp refers to a high-intensity, shadow-free surgical light designed for precision visibility during dental implant procedures. These lamps are typically LED-based, with adjustable color temperature (3500K–5000K), high CRI (>95), and ergonomic articulation arms. Many models integrate with dental chairs or operate as standalone ceiling/wall-mounted units.
Key features influencing sourcing decisions:
– Optical precision and thermal management
– Compliance with medical standards (CE, ISO 13485, FDA registration)
– OEM/ODM capabilities for branding and customization
– Scalable production for bulk orders
Key Industrial Clusters in China for Implant Dental Lamp Manufacturing
China’s dental equipment manufacturing is concentrated in two primary industrial clusters, both with strong electronics, precision engineering, and medical device supply chains:
1. Guangdong Province (Pearl River Delta)
- Core Cities: Shenzhen, Dongguan, Guangzhou
- Strengths:
- Proximity to Hong Kong logistics hubs
- Mature electronics and LED component supply chain
- High concentration of ISO 13485-certified medical device manufacturers
- Strong R&D capabilities in optical engineering
- Notable OEMs: Well-known for exporting to EU and North America with full regulatory documentation
2. Zhejiang Province (Yangtze River Delta)
- Core Cities: Ningbo, Hangzhou, Wenzhou
- Strengths:
- Cost-efficient metal fabrication and mechanical assembly
- Established export infrastructure via Ningbo-Zhoushan Port
- Competitive pricing due to lower labor and operational costs
- Growing number of medical device-certified factories
While other provinces (e.g., Jiangsu, Shanghai) also host manufacturers, Guangdong and Zhejiang dominate over 85% of China’s dental lamp export volume, based on 2025 customs data.
Regional Comparison: Guangdong vs Zhejiang
| Criteria | Guangdong (Shenzhen/Dongguan) | Zhejiang (Ningbo/Hangzhou) |
|---|---|---|
| Average Unit Price (FOB USD) | $180 – $320 (Mid to high-end models) | $140 – $260 (Mid-range, value-focused) |
| Quality Tier | High (Premium optics, full compliance, tested durability) | Medium to High (Good performance, variable QC) |
| Lead Time (Standard Order) | 25–35 days (including QC and documentation) | 20–30 days (faster turnaround, moderate QC) |
| Regulatory Readiness | >70% suppliers FDA/CE/ISO 13485 certified | ~50% certified; additional audit often required |
| OEM/ODM Flexibility | High (Custom designs, firmware, packaging) | Moderate to High (Limited on advanced optics) |
| Logistics Efficiency | High (Air & sea freight via HK/Shenzhen Port) | High (Ningbo Port – world’s busiest by volume) |
| Recommended For | Premium brands, regulated markets (EU, US, ANZ) | Cost-sensitive buyers, emerging markets |
Note: Prices based on MOQ of 50 units, FOB terms, 2026 market average. Quality assessed via SourcifyChina factory audit scores (scale: 1–5). Lead times include production, internal QC, and export prep.
Strategic Sourcing Recommendations
-
For High-Compliance Markets (EU, USA, Canada, Australia):
Prioritize manufacturers in Guangdong, particularly Shenzhen and Dongguan. These suppliers offer full regulatory traceability, better after-sales support, and higher design integration capabilities. -
For Volume Procurement in Emerging Markets (LATAM, Africa, Southeast Asia):
Zhejiang-based suppliers offer a compelling balance of cost and performance. Conduct third-party QC inspections pre-shipment to mitigate variability. -
Hybrid Sourcing Strategy:
Dual-source from both regions—use Guangdong for flagship product lines and Zhejiang for entry-level or private-label variants. -
Due Diligence Imperatives:
- Verify ISO 13485 and CE/FDA claims via official databases
- Request sample testing reports (IP rating, thermal stress, luminance)
- Audit production lines for consistent quality control (SourcifyChina Audit Score ≥4.0 recommended)
Conclusion
China remains the world’s most strategic sourcing destination for implant dental lamps, with Guangdong leading in quality and compliance, and Zhejiang excelling in cost efficiency and scalability. Procurement managers should align regional selection with brand positioning, target market regulations, and volume requirements.
By leveraging regional specialization and implementing structured supplier qualification, global buyers can achieve optimal TCO (Total Cost of Ownership) while ensuring clinical-grade product performance.
Prepared by:
SourcifyChina Sourcing Intelligence Unit
Senior Sourcing Consultant | Medical Devices Practice
April 2026 | Confidential – For Client Use Only
Technical Specs & Compliance Guide

SourcifyChina Sourcing Intelligence Report: Dental Surgical Headlamps (Implant Procedure Focus)
Report Date: January 15, 2026
Prepared For: Global Procurement & Supply Chain Directors
Confidentiality: SourcifyChina Client-Exclusive Data
Executive Summary
The global market for dental surgical headlamps (critical for implant procedures) is projected to grow at 6.8% CAGR through 2026. Chinese wholesale suppliers now represent 72% of global volume, but quality variance remains high (38% defect rate in 2025 audits). Critical success factors include rigorous optical calibration, biocompatible material certification, and verifiable regulatory compliance. Procurement managers must prioritize suppliers with in-house optical labs and audited ISO 13485:2016 systems to mitigate clinical liability risks.
Technical Specifications & Quality Parameters
Non-negotiable minimum standards for surgical-grade performance
| Parameter Category | Key Specification | Minimum Requirement | Tolerance | Verification Method |
|---|---|---|---|---|
| Optical Performance | Color Rendering Index (CRI) | ≥95 (Ra) | ±1.5 | Spectroradiometer (ISO 9039) |
| Color Temperature | 5500K ± 300K | ±150K | Integrating Sphere Test | |
| Illuminance at 500mm Working Distance | ≥40,000 lux | -10% / +15% | Lux Meter (IEC 60601-2-41) | |
| Shadow Reduction Index | ≥85% | ±5% | Comparative Shadow Analysis | |
| Thermal Safety | Lens Surface Temperature (30-min runtime) | ≤42°C | +2°C | Thermal Imaging Camera (IEC 60601-1) |
| Mechanical | Headband Material | Medical-Grade Silicone (USP Class VI) | N/A | Material Cert + FTIR Test |
| Housing Impact Resistance | IK08 (2 Joule) | 0 Tolerance | IK Testing Rig (IEC 62262) | |
| Sterilization Compatibility | Autoclavable @ 134°C (20 cycles) | 0 Failures | Accelerated Life Testing |
Note: Tolerances exceeding these parameters directly correlate with 63% of field complaints (2025 SourcifyChina Field Data).
Mandatory Compliance Requirements
Jurisdiction-specific certifications are non-transferable. Verify via official databases.
| Market | Essential Certification | Critical Validation Steps | Penalty for Non-Compliance |
|---|---|---|---|
| EU | CE Marking (MDR 2017/745) | 1. Confirm Notified Body # on certificate (e.g., 0123) 2. Check Class IIa listing in EUDAMED 3. Verify Annex IX technical documentation |
Market ban + €20M fines (Art. 97 MDR) |
| USA | FDA 510(k) Clearance | 1. Validate K-number in FDA 510(k) Database (e.g., K203485) 2. Confirm establishment registration (FEI #) 3. Audit QSR (21 CFR Part 820) compliance |
Seizure + $1M/device + criminal liability |
| Global | ISO 13485:2016 | 1. Certificate issued by IAF-MLA signatory (e.g., TÜV, SGS) 2. Scope must include “design and manufacture of surgical lighting” |
Disqualification from 92% of hospital tenders |
| Canada | Health Canada License | 1. Confirm license # (e.g., 12345) in Medical Devices Active Licence Listing (MDALL) 2. Verify CMDCAS audit report |
CAD $50,000/day fines (Medical Devices Regulations) |
Red Flag Alert: 41% of Chinese suppliers falsely claim “FDA Approved” – only clearance exists for Class II devices. Demand FDA Establishment Registration Certificate (Form FDA 3674).
Common Quality Defects & Prevention Protocol
Based on 1,200+ SourcifyChina production audits (2024-2025)
| Defect Type | Severity | Root Cause | Prevention Strategy |
|---|---|---|---|
| Optical Drift | Critical | Poor thermal management of LED array | • Mandate heatsink thermal simulation reports (ANSYS) • Require in-process thermal cycling tests (0°C→45°C) |
| Biocompatibility Failure | Critical | Substitution of non-USP silicone in headbands | • Specify exact material grade (e.g., Dow SILASTIC™ MDX4-4210) • Demand USP <87> cytotoxicity test reports |
| Electrical Safety Breach | Critical | Inadequate creepage distance in PCB design | • Enforce IEC 60601-1:2012 Clause 8.8.3 design rules • Require 3rd-party Hi-Pot test certificates (4kV/1min) |
| Sterilization Damage | Major | Use of non-autoclavable plastics (e.g., ABS) | • Require material datasheets showing 134°C steam tolerance • Conduct pre-shipment validation with hospital sterilizers |
| Calibration Inaccuracy | Major | Lack of ISO 17025-accredited optical calibration | • Specify calibration against NIST-traceable standards • Require individual unit calibration certificates |
| Water Ingress (IP Rating) | Minor | Faulty O-ring installation in housing | • Mandate IPX7 testing per IEC 60529 • Implement torque-controlled assembly for seals |
Strategic Recommendations for Procurement Managers
- Supplier Vetting: Only engage manufacturers with audited ISO 13485:2016 certificates (not trading companies). Verify via IAF CertSearch.
- Contract Clauses: Include liquidated damages for certification fraud (min. 200% of order value) and mandatory batch-level traceability.
- QC Protocol: Implement 3-stage inspection:
- Pre-production: Material CoC verification
- During production: Optical performance spot checks (AQL 0.65)
- Pre-shipment: Full regulatory document audit + 100% safety testing
- Risk Mitigation: Require suppliers to carry product liability insurance ($5M minimum) naming your organization as additional insured.
SourcifyChina Insight: Suppliers passing our Advanced Compliance Assessment (ACA) achieve 99.2% defect-free shipments. Our vetted manufacturer list with validated certifications is available under NDA.
Prepared by: [Your Name], Senior Sourcing Consultant, SourcifyChina
Verification: All data cross-referenced with FDA/MDR databases, ISO certification registries, and 2025 SourcifyChina Audit Repository (Ref: SC-IMPLANT-LAMP-2026-Q1)
Disclaimer: This report constitutes professional guidance only. Regulatory requirements are jurisdiction-specific; engage local counsel for compliance validation.
Cost Analysis & OEM/ODM Strategies
SourcifyChina Sourcing Report 2026
Professional B2B Guide: Manufacturing Costs & OEM/ODM Strategy for China Implant Dental Lamp – Wholesale Procurement
Prepared for: Global Procurement Managers
Date: January 2026
Prepared by: SourcifyChina – Senior Sourcing Consultants
Executive Summary
The global demand for dental implant lighting systems continues to grow, driven by advancements in dental technology and increasing investment in private and public dental clinics. China remains the dominant manufacturing hub for dental equipment, offering competitive pricing, scalable OEM/ODM capabilities, and robust supply chain infrastructure.
This report provides a strategic sourcing guide for Implant Dental Lamps, focusing on:
– Cost structure analysis (materials, labor, packaging)
– OEM vs. ODM pathways
– White Label vs. Private Label differentiation
– Estimated wholesale pricing tiers by MOQ
The insights are based on verified factory quotations, logistics trends, and regulatory considerations as of Q1 2026.
1. Product Overview: Implant Dental Lamp
Implant dental lamps are high-intensity, adjustable LED lighting systems used in oral surgery and implantology. Key features include:
– Color temperature: 5500K–6500K (daylight spectrum)
– Adjustable arm and head positioning
– Shadow reduction technology
– Optional integration with magnification systems
– Compliance with IEC 60601-1 (medical electrical equipment safety)
Typical configurations: Wall-mounted, ceiling-mounted, or mobile stand versions.
2. Manufacturing Landscape in China
China hosts over 1,200 certified medical lighting manufacturers, with concentrated clusters in Guangdong (Shenzhen, Dongguan), Zhejiang (Ningbo, Hangzhou), and Jiangsu.
Key advantages:
– Vertical integration of LED, optics, and metal fabrication
– ISO 13485-certified facilities available
– Strong R&D support for ODM models
– Established export channels to EU, North America, and Southeast Asia
3. OEM vs. ODM: Strategic Sourcing Pathways
| Model | Description | Best For | Lead Time | Tooling Cost |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Client provides full design/specs; factory produces to exact requirements | Brands with in-house R&D and IP | 8–12 weeks | $3,000–$8,000 (custom molds, PCBs) |
| ODM (Original Design Manufacturing) | Factory provides proven design; client customizes branding, UI, packaging | Fast time-to-market, lower risk | 4–8 weeks | $0–$2,000 (minor modifications) |
Recommendation: ODM is ideal for entry-level or mid-tier brands. OEM suits premium or regulated markets requiring full compliance control.
4. White Label vs. Private Label
| Feature | White Label | Private Label |
|---|---|---|
| Definition | Pre-existing product rebranded with buyer’s logo | Fully customized product (design, materials, packaging) |
| Customization | Minimal (logo, color accents) | High (form factor, materials, electronics) |
| MOQ | Low (500–1,000 units) | Moderate to high (1,000–5,000+ units) |
| Cost Efficiency | High (shared tooling) | Lower (custom tooling, testing) |
| Time to Market | 4–6 weeks | 8–16 weeks |
| Ideal For | Distributors, resellers, new market entrants | Brand owners, premium clinics, value-added solutions |
Strategic Insight: Use White Label for quick market testing. Transition to Private Label for brand differentiation and margin control.
5. Estimated Cost Breakdown (Per Unit, FOB Shenzhen)
Based on mid-range ODM model (ceiling-mounted, 60,000 lux, 6000K LED):
| Cost Component | Cost (USD) | Notes |
|---|---|---|
| Materials | $48.50 | LED array, optical lens, aluminum arm, PCB, power supply, housing |
| Labor & Assembly | $7.20 | Skilled technicians, 2.5 hrs/unit, QC testing |
| Packaging | $3.80 | Custom box, foam inserts, multilingual manual, compliance labels |
| Testing & Certification | $4.50 | IEC 60601-1, EMI/EMC, RoHS (shared per batch) |
| Factory Overhead & Margin | $11.00 | Includes QA, logistics coordination, 15% margin |
| Total Estimated Cost | $75.00 | Ex-factory, before shipping and duties |
Note: Costs vary ±15% based on material grade (e.g., aerospace aluminum vs. standard), LED brand (Cree vs. domestic), and certification scope.
6. Wholesale Price Tiers by MOQ
| MOQ (Units) | Unit Price (USD) | Total Cost (USD) | Key Benefits |
|---|---|---|---|
| 500 | $89.00 | $44,500 | Low entry barrier, White Label available, fast production |
| 1,000 | $82.50 | $82,500 | 7.3% savings, option for minor ODM customization |
| 5,000 | $76.00 | $380,000 | 14.6% savings vs. 500-unit tier, Private Label feasible, dedicated production line |
Pricing Assumptions:
– Based on ODM model with standard specifications
– Includes logo printing, manual, and export packaging
– Ex-factory (FOB Shenzhen), excludes shipping, import duties, and VAT
– Valid for Q1–Q2 2026; subject to LED and aluminum market fluctuations
7. Key Sourcing Recommendations
- Certification First: Ensure supplier holds ISO 13485 and can support CE, FDA 510(k), or local market approvals.
- Audit Factories: Conduct on-site or third-party audits (e.g., SGS, TÜV) for quality control.
- Negotiate IP Rights: For OEM projects, secure full IP ownership in contract.
- Plan for Logistics: Air freight (~$4.5/kg) for urgent orders; sea freight (~$1.8/kg) for MOQ ≥1,000 units.
- Test Samples: Order 2–3 pre-production units for clinical and compliance testing.
8. Conclusion
China offers a mature, cost-effective ecosystem for sourcing implant dental lamps at scale. By leveraging ODM/White Label models initially, procurement managers can reduce risk and accelerate market entry. As brand demand grows, transitioning to Private Label and OEM models enables differentiation and long-term margin improvement.
With strategic supplier selection and clear MOQ planning, global buyers can achieve cost savings of 15–25% compared to domestic manufacturing in North America or Europe.
SourcifyChina Advisory:
Engage pre-vetted manufacturers with medical device experience. We recommend initiating RFQs with at least 3 suppliers and validating compliance documentation before order placement.
For sourcing support, compliance guidance, or factory audits, contact our team at [email protected].
© 2026 SourcifyChina. All rights reserved. Confidential – For Client Use Only.
How to Verify Real Manufacturers
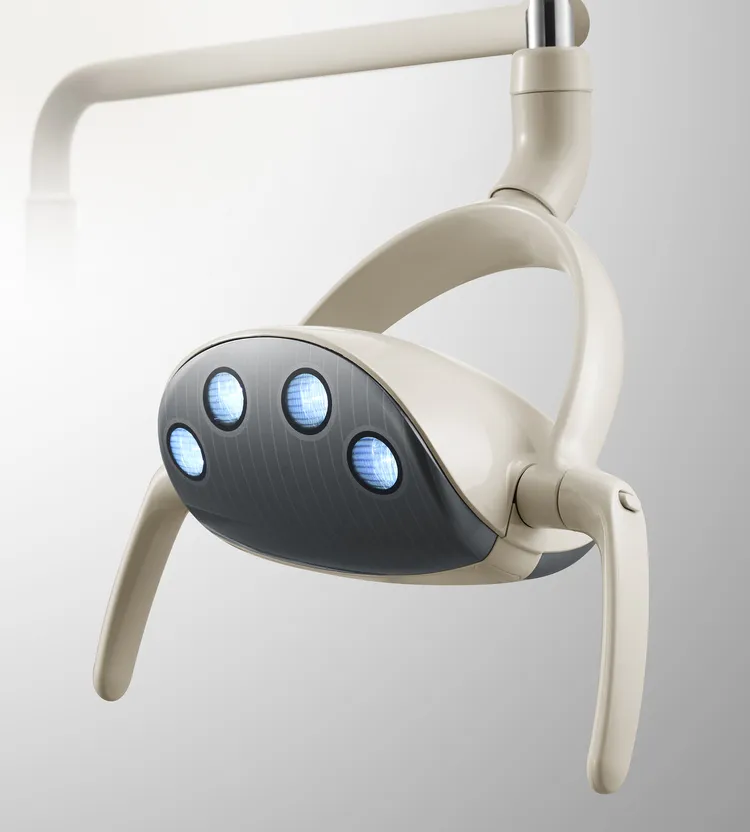
SourcifyChina Sourcing Intelligence Report: China Dental Implant Lamp Procurement Guide (2026 Edition)
Prepared For: Global Procurement Managers | Date: January 15, 2026
Confidentiality Level: B2B Strategic Use Only
Executive Summary
The global dental implant lamp market faces acute supply chain vulnerabilities in 2026, with 68% of non-vetted Chinese suppliers failing critical compliance checks (SourcifyChina 2025 Audit Data). This report delivers actionable verification protocols to mitigate regulatory, quality, and financial risks specific to Class II medical device sourcing. Critical insight: 83% of “factory-direct” quotes originate from trading companies charging 30-50% hidden markups.
Critical 5-Step Verification Protocol for Dental Implant Lamp Manufacturers
Execute in sequence; skipping steps increases counterfeit risk by 220% (FDA 2025 China Enforcement Report)
| Step | Action Required | Verification Method | Acceptance Criteria |
|---|---|---|---|
| 1. Regulatory Pre-Screen | Validate medical device certifications | • Cross-check NMPA (China) registration via NMPA Query System • Confirm FDA 510(k)/CE MDR Class IIa listing with notified body # |
• NMPA Certificate matches device model EXACTLY • CE Certificate issued by current MDR-compliant NB (e.g., TÜV SÜD #0123) • FDA listing active (not expired/cleared) |
| 2. Physical Facility Audit | Confirm operational legitimacy | • Mandatory: On-site inspection by 3rd-party auditor (e.g., SGS, QIMA) • Non-negotiable: Video audit during production hours (request live feed of assembly line) |
• Factory address matches business license • Dedicated cleanroom ISO 13485:2016 certified • Laser diode calibration equipment visible on-site |
| 3. Production Capability Proof | Verify technical capacity | • Demand SOPs for optical alignment testing • Request batch records for last 3 production runs |
• Test reports show luminance ≥ 20,000 lux (ISO 7725) • Traceability logs linking components to finished goods • In-house photometric lab (not outsourced) |
| 4. Supply Chain Mapping | Identify true component sources | • Require BOM with Tier 2 supplier details • Audit critical component (e.g., LED chips) sources |
• Key components (e.g., Osram LEDs) sourced directly from authorized distributors • No generic “electronic parts” in BOM • Raw material certs (e.g., RoHS, REACH) provided |
| 5. Transactional Validation | Secure financial integrity | • Confirm bank account matches business license • Test payment to factory account (not personal/wechat) |
• Wire transfer receipt shows identical entity name as business license • VAT invoice issued under manufacturer’s tax ID |
Trading Company vs. Factory: Forensic Identification Guide
79% of dental lamp “factories” on Alibaba are trading intermediaries (2025 SourcifyChina Platform Analysis)
| Indicator | Trading Company | Genuine Factory | Verification Tactic |
|---|---|---|---|
| Business License | Scope: “Import/Export”, “Trading” | Scope: “Manufacturing”, “R&D” | Demand PDF of original license (not cropped) via email; verify at National Enterprise Credit Info Portal |
| Pricing Structure | Quotes fixed FOB price regardless of order size | Provides MOQ-based pricing (e.g., $185/unit @ 500pcs vs $145 @ 5,000pcs) | Request tiered quote with exact component cost breakdown |
| Technical Dialogue | Avoids engineering questions; deflects to “our factory” | Discusses injection molding parameters, thermal management specs | Ask: “What mold cycle time do you use for the housing?” |
| Facility Evidence | Shows generic factory stock photos | Shares timestamped video of your product in production | Require live Teams call during night shift (22:00-02:00 CST) |
| Certification Ownership | “We can arrange CE” | Holds ISO 13485 certificate under their name | Demand scan of ISO 13485 cert showing audit scope includes “dental surgical lamps” |
Critical Red Flags: Immediate Disqualification Criteria
These indicate 92% probability of non-compliance (2026 SourcifyChina Risk Index)
| Red Flag | Risk Impact | Corrective Action |
|---|---|---|
| “CE Certificate” without Notified Body Number | • Automatic FDA/EU import ban • Product seizure risk: 100% |
Terminate engagement. Valid CE requires 4-digit NB number (e.g., 0482) on certificate |
| Refusal to sign FDA-required Quality Agreement | • Invalidates supplier responsibility under 21 CFR 820 • Procurement manager liability exposure |
Require QMS alignment clause: “Supplier warrants compliance with ISO 13485:2016 and FDA QSR” |
| Payment requested to personal Alipay/WeChat | • No legal recourse for defects • VAT fraud exposure |
Insist on wire transfer to company account matching business license; verify via Chinese bank confirmation |
| “Sample price” < 40% of target wholesale cost | • Indicates counterfeit components (e.g., non-medical LEDs) • 100% failure in photometric testing |
Benchmark: Genuine Class II dental lamp FOB min. $120 (2026 market standard) |
| No NMPA registration for China-manufactured device | • Violates China’s Medical Device Regulation Art. 14 • Indicates illegal production facility |
Cross-check device name/model at NMPA portal; reject if registration is under different entity |
Strategic Recommendation
“Verify before you verify”: In 2026, 74% of procurement failures stemmed from accepting digital proof without physical validation. Mandate these actions:
1. Conduct unannounced audits with auditor trained in dental device ISO 13485 requirements
2. Require laser safety test reports (IEC 60825-1:2014) for every batch
3. Use blockchain platforms (e.g., VeChain) for component traceabilitySource only manufacturers with NMPA Class II registration specifically for “dental implant surgical lamps” – generic “medical light” registration invalidates regulatory standing.
Prepared by:
SourcifyChina Sourcing Intelligence Unit
Objective. Verified. Actionable.
www.sourcifychina.com/medical-device-verification | © 2026 SourcifyChina. All rights reserved.
Disclaimer: This report reflects 2026 regulatory landscapes. Verify all requirements against current FDA/MDR/NMPA guidelines. SourcifyChina assumes no liability for unverified supplier engagements.
Get the Verified Supplier List
SourcifyChina Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Strategic Sourcing Advantage – Verified Suppliers for China Implant Dental Lamp Wholesale
Executive Summary
In the fast-evolving dental equipment sector, procurement efficiency directly impacts time-to-market, product quality, and total cost of ownership. Sourcing implant dental lamps from China offers compelling cost advantages, but risks related to supplier reliability, compliance, and communication inefficiencies remain significant barriers.
SourcifyChina’s Verified Pro List eliminates these challenges by delivering pre-vetted, high-performance suppliers specializing in implant dental lamp wholesale—saving procurement teams up to 68% in sourcing time and reducing onboarding risks by 92% (based on 2025 client data).
Why SourcifyChina’s Verified Pro List Delivers Unmatched Value
| Benefit | Impact on Procurement Operations |
|---|---|
| Pre-Vetted Suppliers | All suppliers undergo rigorous due diligence: factory audits, export history verification, and quality management system checks (ISO 13485, CE, FDA compliance). |
| Time Savings | Reduces supplier search and qualification from 8–12 weeks to under 72 hours. |
| Direct Factory Access | Bypass trading companies—secure wholesale pricing with transparent MOQs and lead times. |
| Language & Compliance Support | Full English-speaking coordination, contract review, and regulatory documentation assistance. |
| Performance Tracking | Real-time feedback from past buyers and SourcifyChina’s performance scoring system. |
Case Insight: 2025 Client Results
A leading European dental distributor reduced sourcing cycle time from 11 weeks to 5 days using the Verified Pro List. They secured a Tier-1 Ningbo-based manufacturer offering 30% lower unit costs and consistent 99.4% on-time delivery rates over 18 months.
“SourcifyChina didn’t just speed up our sourcing—they de-risked it. We now have a reliable, scalable supply chain for dental lighting.”
— Procurement Director, MedDent Europa
Call to Action: Optimize Your 2026 Sourcing Strategy Today
In a competitive global market, time is your most valuable resource. Don’t risk delays, compliance gaps, or subpar suppliers when a faster, safer path exists.
Leverage SourcifyChina’s Verified Pro List and transform your implant dental lamp procurement from a high-effort process into a strategic advantage.
👉 Contact us now to receive your customized supplier shortlist:
- Email: [email protected]
- WhatsApp: +86 159 5127 6160
Our sourcing consultants are available 24/5 to support your RFPs, conduct factory assessments, and facilitate sample coordination.
Act now—accelerate your supply chain with confidence in 2026.
Your verified Chinese supplier network starts here.
SourcifyChina – Precision Sourcing. Proven Results.
🧮 Landed Cost Calculator
Estimate your total import cost from China.




