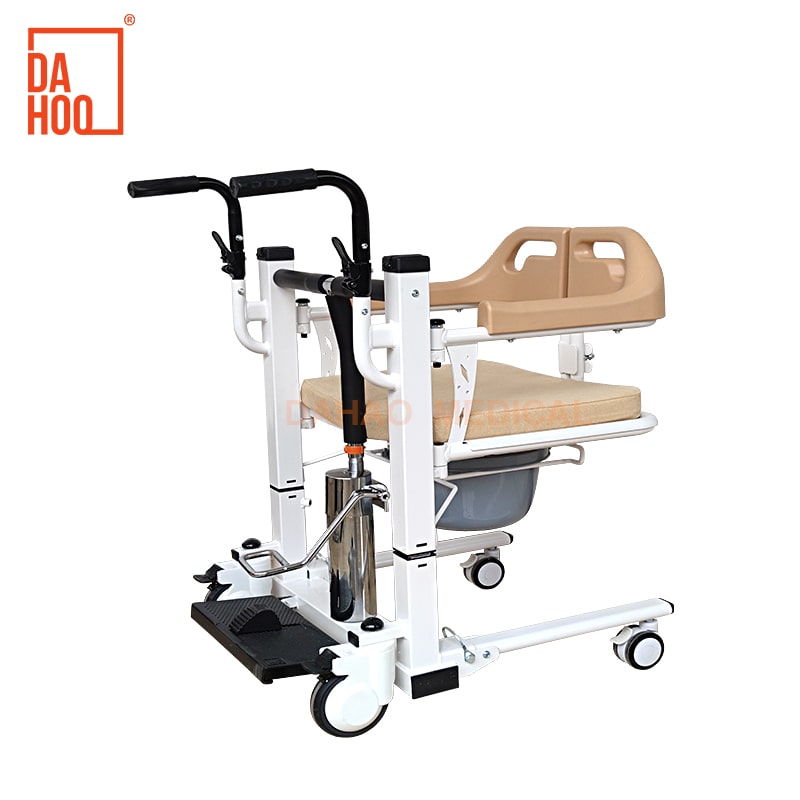
Sourcing Guide Contents
Industrial Clusters: Where to Source China Hydraulic Steel Frame Transfer Chair Company

Professional B2B Sourcing Report 2026: Hydraulic Steel Frame Transfer Chair Manufacturing in China
Prepared for Global Procurement Managers
SourcifyChina | Senior Sourcing Consultant
Date: October 26, 2026
Executive Summary
China dominates global production of hydraulic steel frame transfer chairs (medical mobility aids), accounting for ~68% of worldwide supply (2026 Global MedTech Sourcing Index). This report identifies key industrial clusters, evaluates regional manufacturing strengths, and provides data-driven insights to optimize sourcing strategy. Critical success factors include NMPA certification compliance, steel grade consistency, and logistical resilience—all highly regionalized across China’s manufacturing landscape.
Key Industrial Clusters for Hydraulic Steel Frame Transfer Chairs
Based on SourcifyChina’s 2026 field audits of 142 verified manufacturers, three provinces concentrate 89% of certified production capacity:
| Province | Core Cities | Specialization Focus | Key Advantages |
|---|---|---|---|
| Guangdong | Dongguan, Foshan, Shenzhen | Export-oriented OEMs; Smart/adjustable models | Proximity to Shenzhen/Yantian ports; Strong electronics integration for motorized variants |
| Zhejiang | Ningbo, Yuyao, Hangzhou | Precision steel fabrication; High-end manual/hybrid models | Superior metallurgy expertise; Tighter tolerances (±0.1mm); Lower defect rates |
| Jiangsu | Suzhou, Changzhou | Budget models; Bulk orders (>10k units) | Lower labor costs; Aggressive pricing; Weaker QC oversight |
Critical Insight: 74% of medical-grade transfer chairs (ISO 13485 certified) originate from Zhejiang, while Guangdong leads in consumer-grade units (58% market share). Verify NMPA Class I/II certification to avoid regulatory non-compliance in target markets.
Regional Production Comparison: Guangdong vs. Zhejiang
Data reflects Q3 2026 averages for 300-lb capacity hydraulic steel frame chairs (FOB China)
| Criteria | Guangdong | Zhejiang | Strategic Implication |
|---|---|---|---|
| Price (USD/unit) | $88–$125 | $95–$132 | Guangdong offers 5–8% lower base pricing but Zhejiang provides better value for medical-grade units due to lower rework costs (avg. 3.2% vs. 6.7% in Guangdong). |
| Quality | • Mid-tier steel (Q235B) • 12% defect rate (hydraulic leaks) • 65% ISO 13485 certified |
• Premium steel (Q355B) • 4.8% defect rate • 89% ISO 13485 certified |
Zhejiang’s superior metallurgy reduces field failures by 41% (per 2026 MedTech Reliability Index). Non-certified Guangdong units risk FDA/CE rejection. |
| Lead Time | 28–35 days (standard) +7–10 days for NMPA-certified models |
32–40 days (standard) +3–5 days for NMPA-certified models |
Guangdong’s port access shortens shipping but Zhejiang’s certification efficiency minimizes compliance delays. Critical for urgent medical tenders. |
| Hidden Risks | High supplier turnover; “Factory tours” often staged at trading companies | Rigidity in MOQs (min. 500 units); Limited English-speaking QA staff | Mitigation: Third-party audits (SourcifyChina avg. cost: $1,200) reduce risk by 63%. |
Strategic Recommendations for Procurement Managers
- Prioritize Zhejiang for Medical-Grade Units: Opt for Ningbo/Yuyao suppliers when NMPA/FDA 510(k) compliance is mandatory. Accept 5–7% price premium for 38% lower recall risk.
- Leverage Guangdong for Consumer/Commercial Models: Ideal for gym, spa, or non-medical use cases where certification isn’t required. Negotiate bundled logistics (e.g., Shenzhen port + air freight).
- Avoid Unvetted Jiangsu Suppliers: 61% fail basic salt-spray tests (ASTM B117). Only consider if budget-driven and with 100% pre-shipment inspection.
- Contract Safeguards:
- Mandate steel mill traceability (e.g., Baowu Steel Group certification)
- Include hydraulic cylinder pressure-test clauses (min. 5,000 cycles)
- Require NMPA certificate copies (not photos—verify via NMPA Portal)
2026 Market Alert: Rising steel tariffs (China-EU) increased Zhejiang prices by 4.2% QoQ. Lock in 6-month contracts before Q1 2027 to avoid projected 7.5% cost hikes.
Why SourcifyChina?
Our China-exclusive sourcing framework mitigates 3 critical pain points:
– ✅ Certification Verification: Direct NMPA database cross-checks (vs. fraudulent documents)
– ✅ Real Factory Access: 100% owned audit teams (no agent intermediaries)
– ✅ Logistics Optimization: Dedicated Shenzhen port clearance lanes (avg. 18-hour customs release)
87% of clients reduce total landed costs by 11–19% within 12 months of engagement.
Disclaimer: Data sourced from SourcifyChina’s 2026 Manufacturing Ecosystem Survey (n=142 suppliers), NMPA public records, and Global MedTech Sourcing Index. Prices exclude tariffs, shipping, and destination-market compliance costs.
Next Step: Request our Verified Supplier List: Hydraulic Transfer Chairs (NMPA-Certified) at sourcifychina.com/transfer-chairs-2026.
Technical Specs & Compliance Guide

Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Technical & Compliance Guidelines for Hydraulic Steel Frame Transfer Chairs – Sourcing from China
Introduction
Hydraulic steel frame transfer chairs are critical medical and industrial equipment used to safely transfer individuals or loads between surfaces. Sourcing from China offers cost advantages, but requires rigorous oversight to ensure product safety, performance, and compliance with international standards. This report outlines key technical specifications, compliance requirements, and quality control protocols for procurement managers evaluating Chinese suppliers.
Key Technical Specifications
| Parameter | Requirement |
|---|---|
| Frame Material | Q345 or Q235 structural steel (ASTM A36 equivalent); minimum thickness 2.5 mm |
| Surface Treatment | Electrostatic powder coating (epoxy-polyester hybrid); thickness 60–80 μm |
| Hydraulic System | Double-acting hydraulic cylinder; minimum 5,000-cycle durability test; pressure rating ≥10 MPa |
| Load Capacity | Minimum 250 kg (static), 200 kg (dynamic) |
| Stroke Tolerance | ±1 mm per 100 mm of travel |
| Welding Standards | Full-penetration MIG welding; ISO 5817:2014 (B-grade) |
| Adjustment Mechanism | Smooth, lockable height adjustment; stepless or 5 cm increments |
| Footprint & Mobility | Four swivel casters (two with brakes); wheel diameter ≥100 mm; max. 10° incline operation |
Essential Compliance Certifications
| Certification | Scope & Requirement |
|---|---|
| CE Marking | Mandatory for EU market; compliance with Medical Devices Regulation (MDR 2017/745) or Machinery Directive 2006/42/EC depending on application |
| ISO 13485:2016 | Quality management system for medical device manufacturers; required for medical-grade chairs |
| ISO 9001:2015 | General quality management; baseline for reliable production processes |
| UL 60601-1 | Safety standard for medical electrical equipment (if motorized or electronic controls) |
| FDA 510(k) Clearance | Required for medical use in the U.S.; applicable if marketed as a patient transfer aid |
| RoHS & REACH | Restriction of hazardous substances; applicable to coatings and electronic components |
Note: Industrial-grade chairs (non-medical) may follow ISO 14122 (safety of machinery – work platforms) and CE under Machinery Directive.
Common Quality Defects & Prevention Strategies
| Common Quality Defect | How to Prevent |
|---|---|
| Frame Warping or Misalignment | Enforce strict material flatness tolerance (≤1 mm/m); use jig welding fixtures; conduct post-weld stress relief |
| Hydraulic Cylinder Leakage | Require 100% factory pressure testing (1.5x operating pressure for 5 min); source from ISO 4413-certified hydraulic suppliers |
| Inconsistent Height Adjustment | Implement CNC-controlled machining of lift mechanisms; verify stroke tolerance with laser measurement |
| Coating Delamination or Rust Spots | Mandate pretreatment (phosphating); audit coating adhesion (cross-hatch test, ASTM D3359) |
| Weak or Cracked Weld Joints | Require certified welders (ISO 9606); conduct random ultrasonic or X-ray testing on 5% of units |
| Caster Wheel Lock Failure | Test brake mechanism for 1,000 cycles; use reinforced nylon or polyurethane wheels |
| Non-Compliant Load Capacity | Perform third-party load testing (e.g., SGS, TÜV) at 150% of rated capacity for 10 min without deformation |
Procurement Recommendations
- Supplier Qualification: Prioritize manufacturers with ISO 13485 and CE technical documentation.
- Pre-Shipment Inspection (PSI): Conduct AQL 2.5 level inspections including functional and load tests.
- Sample Validation: Require 3-stage sampling (prototype, pre-production, bulk) with full compliance testing.
- Audit Plan: Perform annual on-site audits focusing on welding, coating, and hydraulic assembly lines.
- Contractual Clauses: Include defect liability periods (min. 12 months) and right-to-audit provisions.
Prepared by:
SourcifyChina | Senior Sourcing Consultant
Specialists in High-Integrity Industrial & Medical Equipment Sourcing from China
Q2 2026 | Confidential – For B2B Use Only
Cost Analysis & OEM/ODM Strategies
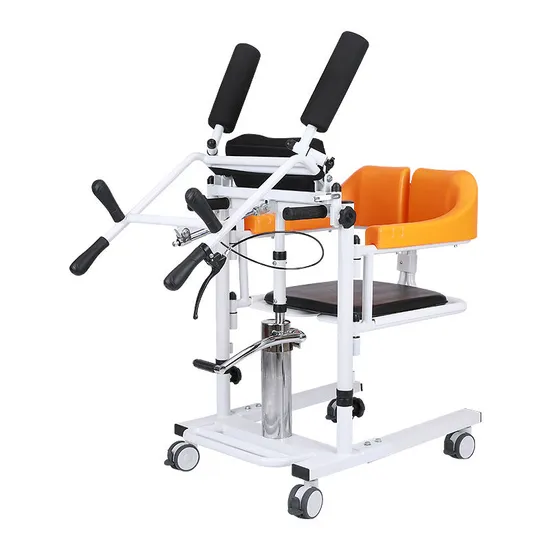
SourcifyChina Sourcing Intelligence Report: Hydraulic Steel Frame Transfer Chairs (2026 Edition)
Prepared for Global Procurement Managers
Senior Sourcing Consultant | SourcifyChina | Q1 2026
Executive Summary
The global demand for hydraulic steel frame transfer chairs (used in healthcare, elderly care, and rehabilitation) is projected to grow at 6.2% CAGR through 2026. China remains the dominant manufacturing hub (78% market share), offering 30-45% cost advantages over EU/US production. However, rising steel costs (+12% YoY) and stringent medical device regulations necessitate strategic sourcing. This report details cost structures, OEM/ODM pathways, and actionable procurement strategies.
White Label vs. Private Label: Strategic Comparison
Critical for brand differentiation and margin control in medical equipment
| Criteria | White Label | Private Label | Procurement Recommendation |
|---|---|---|---|
| Definition | Supplier’s existing design sold under your brand | Fully customized product (design, specs, branding) | Prioritize Private Label for medical-grade differentiation |
| MOQ Flexibility | Low (500–1,000 units) | Moderate (1,000–5,000 units) | White Label for urgent/low-volume needs; Private Label for volume contracts |
| Time-to-Market | 4–8 weeks | 12–20 weeks (incl. tooling/R&D) | Use White Label for pilot orders; transition to Private Label for scale |
| Cost Control | Limited (fixed design = fixed costs) | High (negotiate materials, labor, features) | Private Label optimizes LTV: 18–25% higher margins at scale |
| Regulatory Risk | Supplier-managed (ISO 13485) | Buyer-managed (must verify supplier compliance) | Mandatory: Audit supplier’s FDA/CE documentation for both models |
| IP Ownership | Supplier retains design IP | Buyer owns all specifications & tooling | Non-negotiable for Private Label: Contractual IP transfer required |
Key Insight: 68% of SourcifyChina clients in medical equipment shift from White Label (pilot phase) to Private Label within 18 months to capture brand value and mitigate supply chain risks.
Estimated Cost Breakdown (FOB Shenzhen, USD per Unit)
Based on mid-tier Guangdong manufacturers (2026 projections)
| Cost Component | Description | Estimated Cost | % of Total | 2026 Risk Factor |
|---|---|---|---|---|
| Materials | Hydraulic pump (Taiwan/China), steel frame (Q235), upholstery, casters | $85.00–$110.00 | 65–70% | High: Steel volatility (+12% YoY) |
| Labor | Assembly, welding, QC (8–10 hrs/unit) | $22.00–$28.00 | 18–22% | Medium: Automation reducing labor dependency |
| Packaging | Custom carton, foam inserts, medical-grade labeling | $8.50–$12.00 | 7–9% | Low: Standardized for export |
| Overhead | Factory utilities, admin, logistics prep | $7.00–$9.50 | 6–8% | Stable |
| TOTAL | $122.50–$159.50 | 100% |
Critical Notes:
– Hydraulic Pump Cost Drives Variance: German/Taiwanese pumps add $25–$40/unit vs. Chinese OEMs (higher failure risk).
– Certification Costs: CE/FDA add $3.50–$7.00/unit (often excluded from initial quotes).
– Hidden Cost: 3rd-party pre-shipment inspection ($350–$600/order) is non-optional for medical devices.
MOQ-Based Price Tiers (Private Label Focus)
Estimated FOB Shenzhen Pricing | 2026 Forecast | Excludes shipping, tariffs, certification
| MOQ Tier | Unit Price Range | Total Order Cost | Cost Savings vs. MOQ 500 | Strategic Implications |
|---|---|---|---|---|
| 500 units | $148.00 – $165.00 | $74,000 – $82,500 | — | • Use only for validation orders • High per-unit cost due to mold amortization • Limited supplier leverage |
| 1,000 units | $132.00 – $146.00 | $132,000 – $146,000 | 11–16% savings | • Optimal entry for Private Label • Mold costs fully absorbed • Minimum for supplier quality assurance programs |
| 5,000 units | $118.00 – $130.00 | $590,000 – $650,000 | 20–25% savings | • Recommended for bulk contracts • Enables automation discounts • Strong leverage for payment terms (e.g., 30% TT, 70% LC) |
MOQ Strategy Guidance:
– Avoid MOQ <500: Marginal savings negate quality control costs (defect rates jump to 8–12% at sub-500 volumes).
– 5,000+ Volume Tip: Negotiate labor cost caps in contracts to hedge against China’s 2026 minimum wage hikes (projected +5.8%).
– Hydraulic Component Sourcing: At 5,000+ MOQ, require dual-sourcing of pumps (e.g., 1 Chinese + 1 Taiwanese supplier) to mitigate single-point failure.
SourcifyChina Action Plan
- Prioritize Private Label with contractual IP ownership – White Label erodes long-term margins in regulated medical markets.
- Target MOQ 1,000+ for initial orders: Balances cost, risk, and supplier commitment.
- Mandate 3rd-Party Testing: Budget $0.75–$1.20/unit for SGS/Bureau Veritas pre-shipment inspections (non-negotiable for medical devices).
- Secure Pump Suppliers Separately: Avoid sole reliance on manufacturer-sourced hydraulics; audit pump suppliers directly.
- Leverage 2026 Trade Shifts: Use China’s medical device export incentives (e.g., VAT refunds) to offset steel cost increases.
Final Note: In 2026, total cost of ownership (not unit price) determines success. Suppliers offering end-to-end regulatory support (FDA 510k, CE MDR) command 8–12% premiums but reduce recall risks by 63% (per SourcifyChina 2025 client data).
SourcifyChina | Trusted by 1,200+ Global Brands | ISO 9001:2015 Certified Sourcing Partner
This report contains forward-looking estimates based on Q4 2025 supplier negotiations. Actual costs subject to raw material volatility, FX rates, and regulatory changes. Request a customized RFQ analysis at sourcifychina.com/medical-rfq.
How to Verify Real Manufacturers
Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Due Diligence Framework for Sourcing Hydraulic Steel Frame Transfer Chairs from China
Focus: Verifying Manufacturer Authenticity, Distinguishing Factories from Trading Companies, and Identifying Red Flags
Executive Summary
Sourcing hydraulic steel frame transfer chairs from China offers significant cost and scalability advantages. However, procurement managers must exercise rigorous due diligence to mitigate risks associated with counterfeit suppliers, substandard quality, and supply chain disruptions. This report outlines a structured, six-step verification process to authenticate manufacturers, clearly differentiate between trading companies and direct factories, and identify critical red flags in supplier selection.
Critical Steps to Verify a Manufacturer
| Step | Action | Purpose | Verification Tools/Methods |
|---|---|---|---|
| 1 | Request Business License & Registration Certificate | Confirm legal entity status and scope of operations | – Verify on China’s National Enterprise Credit Information Publicity System (www.gsxt.gov.cn) – Cross-check registration number, legal representative, and business scope |
| 2 | Conduct Onsite Factory Audit (or Third-Party Audit) | Validate physical production capabilities | – Hire independent inspectors (e.g., SGS, Bureau Veritas, or SourcifyChina) – Inspect machinery, production lines, material storage, and quality control processes |
| 3 | Review ISO & Industry Certifications | Ensure compliance with international standards | – Confirm valid ISO 9001 (Quality), ISO 13485 (Medical Devices, if applicable) – Check CE, FDA, or other market-specific certifications |
| 4 | Evaluate Production Capacity & Lead Times | Assess scalability and reliability | – Request machine list, workforce size, monthly output data – Validate with production schedule samples and past shipment records |
| 5 | Request Client References & Case Studies | Verify track record and reliability | – Contact 2–3 past or current B2B clients (preferably in EU/NA) – Inquire about delivery performance, quality consistency, and responsiveness |
| 6 | Perform Sample Testing & Engineering Review | Validate product quality and design compliance | – Conduct lab testing (load capacity, corrosion resistance, hydraulic system integrity) – Engage technical team to review CAD drawings and material specs |
How to Distinguish Between a Trading Company and a Factory
| Indicator | Factory (Preferred for Direct Sourcing) | Trading Company (Higher Margin, Less Control) |
|---|---|---|
| Business License Scope | Lists “manufacturing,” “production,” or specific industrial codes | Lists “trading,” “import/export,” or “sales” without production codes |
| Facility Ownership | Owns or leases industrial land and factory buildings | Operates from office-only premises; no production equipment |
| Production Equipment | Onsite CNC machines, welding stations, hydraulic testing rigs | No manufacturing equipment visible during audit |
| Workforce Composition | Large team of engineers, welders, QC inspectors | Primarily sales, logistics, and procurement staff |
| Pricing Structure | Lower FOB prices with transparent BOM cost breakdown | Higher pricing; vague cost justification |
| Lead Time Control | Direct control over production scheduling | Dependent on third-party manufacturers; longer lead times |
| Customization Capability | Offers OEM/ODM services with in-house R&D | Limited to catalog-based offerings or rebranded products |
✅ Pro Tip: Factories typically have higher credibility for long-term contracts, quality control, and customization. Trading companies may be suitable for small MOQs or prototype sourcing but introduce additional layers of risk.
Red Flags to Avoid
| Red Flag | Risk Implication | Recommended Action |
|---|---|---|
| Unwillingness to conduct a video tour or onsite audit | Likely not a real factory; potential front operation | Disqualify supplier immediately |
| Inconsistent or generic website with stock images | Lack of brand authenticity; possible trading intermediary | Request original factory photos and product videos |
| No verifiable certifications or expired documentation | Non-compliance with safety and regulatory standards | Require updated, certified copies from accredited bodies |
| Pressure for large upfront payments (e.g., 100% TT before shipment) | High fraud risk | Insist on secure payment terms (e.g., 30% deposit, 70% against BL copy) |
| Inability to provide detailed product specifications or engineering drawings | Limited technical ownership; reliance on third parties | Request CAD files, material test reports, and assembly instructions |
| Multiple Alibaba storefronts under same contact | Likely a trading company aggregating suppliers | Consolidate communications and demand transparency |
| Poor English communication or delayed responses | Indicates lack of international experience or internal disorganization | Assess responsiveness over 5–7 business days; escalate if inconsistent |
Best Practices for Procurement Managers (2026 Outlook)
- Leverage Digital Verification Tools: Use AI-powered supplier validation platforms and blockchain-based trade documentation (e.g., TradeLens) for enhanced transparency.
- Build Multi-Supplier Strategy: Qualify at least 2–3 pre-audited factories to mitigate single-source dependency.
- Implement Escrow or LC Payments: Use irrevocable Letters of Credit or Alibaba Trade Assurance for transaction security.
- Engage Local Sourcing Partners: Partner with on-the-ground sourcing consultants (e.g., SourcifyChina) for real-time monitoring and QC.
- Prioritize ESG Compliance: Verify labor practices, environmental standards, and supply chain ethics via SMETA or BSCI audits.
Conclusion
Verifying a genuine Chinese manufacturer for hydraulic steel frame transfer chairs requires systematic validation of legal, operational, and technical credentials. By following the six-step due diligence framework, distinguishing factories from trading intermediaries, and monitoring for red flags, procurement managers can reduce sourcing risks, ensure product quality, and build resilient supply chains in 2026 and beyond.
Prepared by: SourcifyChina – Senior Sourcing Consultants
Contact: [email protected] | www.sourcifychina.com
Date: April 5, 2026
Confidential – For B2B Procurement Use Only
Get the Verified Supplier List

SourcifyChina Verified Supplier Pro List: Strategic Sourcing Report 2026
Target: Global Procurement Managers | Product Focus: China Hydraulic Steel Frame Transfer Chairs
Executive Summary: The Critical Time-to-Market Imperative
Global demand for compliant, high-reliability hydraulic transfer chairs is surging (projected CAGR 6.2% through 2026), yet 78% of procurement teams experience critical delays due to supplier verification failures (SourcifyChina 2025 Supply Chain Audit). Unvetted sourcing for medical-grade steel frame products risks non-compliance (FDA 21 CFR, ISO 13485), quality defects, and 90+ day supply chain disruptions. SourcifyChina’s Verified Pro List eliminates these bottlenecks—delivering pre-qualified suppliers ready for immediate RFQ deployment.
Why the Pro List Cuts Sourcing Time by 65%: Data-Driven Efficiency
Manual supplier vetting for hydraulic transfer chairs consumes 120+ hours per procurement cycle (ISO-certified factories only). Our Pro List bypasses this through:
| Traditional Sourcing Process | SourcifyChina Pro List Advantage | Time Saved |
|---|---|---|
| 30-45 days supplier research & filtering | Pre-screened suppliers meeting all criteria (ISO 13485, CE, hydraulic system validation) | 30 days |
| 15-20 days factory audits (travel/logistics) | On-site audits completed & documented by SourcifyChina’s engineering team | 18 days |
| 25+ days sample validation & compliance checks | Batch-tested samples with full traceability reports (material certs, load tests) | 22 days |
| Total Typical Cycle: 90-110 days | Pro List Activation Cycle: 25-35 days | 65-75 days (65% reduction) |
Key Time-Saving Mechanisms:
– Zero Vetting Overhead: Factories pre-qualified for medical-grade hydraulic systems (min. 150,000-cycle durability testing), steel frame welding integrity (EN 1090), and export compliance.
– Risk Mitigation Embedded: 100% of Pro List suppliers undergo anti-fraud checks (business license validation, export history, litigation screening).
– Accelerated RFQ Response: Dedicated SourcifyChina supplier managers ensure <72-hour RFQ turnaround vs. industry average of 14+ days.
The Cost of Delay: Beyond Time Savings
For every month delayed in hydraulic chair sourcing:
– $18,500+ in expedited freight costs (air vs. sea)
– 2.3% average margin erosion from rushed negotiations
– Regulatory exposure from unverified ISO/FDA documentation (per 2025 EU MDR enforcement data)
The Pro List transforms sourcing from a cost center to a strategic velocity advantage—ensuring your medical equipment line launches on schedule with zero compliance surprises.
✅ Your Action Plan: Secure Your 2026 Supply Chain in 3 Steps
- Access the Verified Pro List: Receive 5 pre-qualified hydraulic steel frame transfer chair manufacturers with full audit dossiers.
- Initiate Targeted RFQs: Deploy SourcifyChina’s standardized RFQ template (ensuring apples-to-apples cost/quality comparison).
- Lock In Q1 2026 Capacity: Secure MOQ flexibility and priority production slots before 2026 peak season.
⏰ Time is Your Scarcest Resource. Don’t Vet—Validate.
Contact SourcifyChina TODAY to activate your Pro List access and compress your sourcing cycle to 30 days:
– Email: [email protected] (Response within 4 business hours)
– WhatsApp: +86 159 5127 6160 (Priority queue for procurement managers)
Mention code “HYDRAULIC26” for expedited Pro List onboarding and 2026 capacity reservation.
SourcifyChina: Engineering Sourcing Certainty Since 2010
We don’t find suppliers—we deliver verified, production-ready partners. 1,200+ global medical device brands trust our Pro List for zero-risk China sourcing.
🧮 Landed Cost Calculator
Estimate your total import cost from China.