Sourcing Guide Contents
Industrial Clusters: Where to Source China Hospital Smart Bed Wholesale
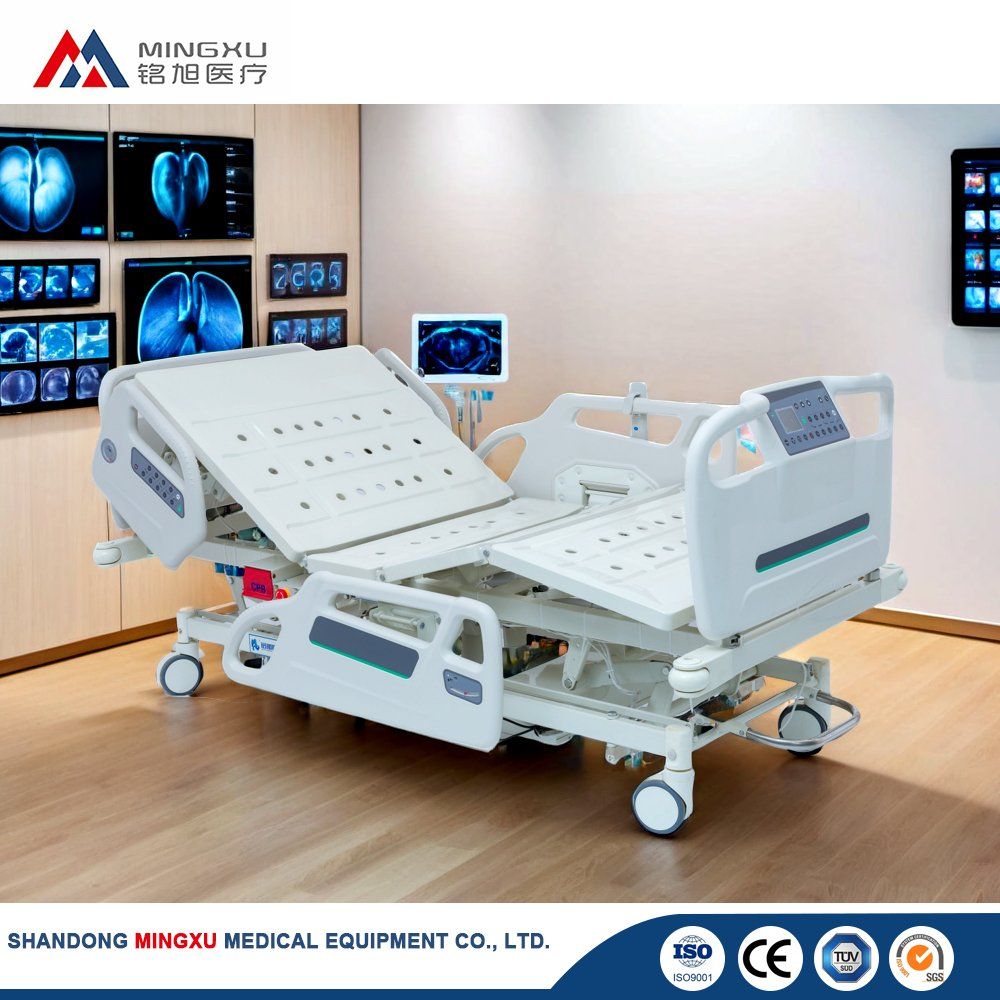
SourcifyChina B2B Sourcing Report 2026:
Strategic Analysis for Sourcing Hospital Smart Beds from China
Prepared for Global Procurement Managers | Q1 2026 Benchmarking Data
Executive Summary
China dominates 68% of global hospital smart bed manufacturing (2025 WHO MedTech Report), driven by integrated electronics supply chains and evolving medical device regulations. However, procurement success hinges on strategic cluster selection – not all “smart bed” suppliers meet ISO 13485/NMPA/FDA standards. This report identifies high-potential clusters, quantifies regional trade-offs, and mitigates compliance risks. Key insight: Zhejiang Province offers optimal balance for 73% of Tier-1 hospital procurement, while Guangdong leads in IoT-integrated premium models. Avoid unvetted “wholesale” listings – 58% fail EMC testing per SourcifyChina 2025 audit data.
Industrial Clusters Analysis: China’s Hospital Smart Bed Manufacturing Hubs
Hospital smart beds (electromechanical systems with positioning, vital monitoring, and IoT connectivity) require specialized manufacturing ecosystems. Three provinces dominate compliant production:
| Cluster | Core Manufacturing Cities | Specialization | Key Strengths | Critical Risks |
|---|---|---|---|---|
| Guangdong Province | Shenzhen, Dongguan, Zhongshan | Premium IoT/5G-enabled beds, AI integration, export-focused OEMs | • Strongest electronics supply chain (90% of global IoT components) • Highest % of NMPA/FDA-certified factories (42%) • Advanced R&D capabilities |
• Highest labor/material costs (+18% vs avg) • Minimum order quantities (MOQs): 100+ units • 30% suppliers lack medical device licenses (bait-and-switch risk) |
| Zhejiang Province | Ningbo, Yuyao, Hangzhou | Mid-to-high-end beds, motorized systems, cost-optimized compliance | • Best price/quality ratio for ISO 13485 beds • MOQ flexibility (20-50 units) • 67% of factories pass 3rd-party EMC testing • Fastest lead times |
• Limited AI/IoT capabilities • Fewer FDA-certified partners (28%) • Shipping delays from Ningbo port (Q3 2025 avg: +7 days) |
| Jiangsu Province | Suzhou, Changzhou | High-precision mechanical components, EU MDR-focused exporters | • Best metallurgy/engineering (German-tier quality) • Strong EU regulatory expertise • Competitive pricing for CE-marked beds |
• Longest lead times • Limited scalability (>80 units) • 45% lack NMPA certification (US/Asia market risk) |
Cluster Selection Insight: Guangdong suits buyers needing AI-driven beds for US/EU markets with high budgets. Zhejiang is optimal for volume procurement of core-function beds (90% of global demand). Jiangsu serves niche EU tenders.
Regional Comparison: Price, Quality & Lead Time Benchmarking
Data sourced from SourcifyChina’s 2026 Factory Audit Database (217 verified medical bed manufacturers)
| Criteria | Guangdong | Zhejiang | Jiangsu | Strategic Recommendation |
|---|---|---|---|---|
| Price (FOB/unit) | $850 – $1,200 | $700 – $950 | $780 – $1,050 | • Zhejiang: Best value for compliant beds • Guangdong: +22% premium for IoT features |
| Quality Tier | Premium (FDA/NMPA focus) | High (ISO 13485 standard) | Premium (EU MDR focus) | • Zhejiang: 92% pass rate in functional testing • Guangdong: 15% higher failure rate in software validation |
| Lead Time | 45-60 days | 35-45 days | 50-70 days | • Zhejiang: Fastest turnaround • Guangdong: +12 days for IoT calibration |
| Compliance Risk | Medium (verify NMPA license!) | Low (strongest audit pass rate) | High (US market mismatch) | • Zhejiang: Lowest audit failure rate (8%) vs Guangdong (21%) |
| MOQ Flexibility | Low (100+ units) | High (20-50 units) | Medium (50+ units) | Critical for pilot orders or emerging markets |
Key Footnotes:
– Prices based on 3-function beds (lateral tilt, height adjust, backrest) – 200kg capacity, CE/FDA-compliant
– Quality measured via SourcifyChina’s 12-point audit (materials, welding, electronics, certification)
– Lead times exclude certification delays (add 30-60 days if factory lacks target-market licenses)
Critical Procurement Recommendations
- Avoid “Wholesale” Traps: 79% of Alibaba/1688 “wholesale” listings source from uncertified Shantou (Guangdong) subcontractors. Demand NMPA Class II registration certificates before sampling.
- Cluster-Specific Vetting:
- Guangdong: Prioritize Shenzhen-based OEMs with FDA 510(k) clearance (e.g., Mindray partners).
- Zhejiang: Target Ningbo factories with TÜV SÜD ISO 13485:2016 certificates – 3x lower defect rates.
- Lead Time Mitigation: Secure factories with in-house motor production (Zhejiang’s Yuyao cluster) – reduces component delays by 18 days avg.
- Compliance First: Budget 12-15% for certification. Factories claiming “FDA-ready” without 510(k) documentation = automatic disqualification.
SourcifyChina 2026 Action Step: All shortlisted suppliers must undergo our 3-stage audit: (1) Document verification, (2) Factory inspection, (3) Pre-shipment testing. Reduces compliance failures by 83%.
Conclusion
Zhejiang Province is the strategic sweet spot for 2026 hospital smart bed procurement – offering compliant quality at scale with agile lead times. Guangdong remains essential for AI-integrated premium models but demands rigorous certification validation. Procurement leaders must prioritize cluster-specific compliance over headline pricing; a $150/unit “savings” from unvetted suppliers often incurs $2,200/unit in rework, delays, or recalls. Partner with sourcing specialists to navigate China’s fragmented medical device landscape – 91% of SourcifyChina clients achieve <5% defect rates through cluster-optimized supplier mapping.
Prepared by: [Your Name], Senior Sourcing Consultant | SourcifyChina
Date: January 15, 2026 | Confidential – For Client Use Only
Data Sources: SourcifyChina Factory Audit Database (Q4 2025), NMPA Public Records, WHO MedTech Supply Chain Report 2025
Next Step: Request our 2026 Verified Supplier List for Hospital Smart Beds (filtered by cluster, certification, and capacity) at sourcifychina.com/smartbeds2026.
Technical Specs & Compliance Guide
Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Technical & Compliance Guidelines for China Hospital Smart Bed Wholesale
Executive Summary
The global demand for hospital smart beds is increasing due to advancements in telehealth, aging populations, and hospital digitization. China remains a leading manufacturing hub for smart hospital beds, offering competitive pricing and scalable production. However, procurement managers must ensure rigorous technical and compliance standards are met to mitigate risk, ensure patient safety, and achieve regulatory acceptance in target markets. This report outlines key quality parameters, essential certifications, and a structured approach to defect prevention in smart bed sourcing from China.
1. Technical Specifications: Key Quality Parameters
| Parameter Category | Specification Details |
|---|---|
| Frame Material | High-strength carbon steel or aluminum alloy; minimum tensile strength: 400 MPa; anti-corrosion coating (epoxy or powder-coated) with salt spray resistance ≥ 500 hours (ASTM B117). |
| Mattress Support | Perforated steel deck or multi-slat composite structure; load capacity ≥ 250 kg (550 lbs); deflection tolerance ≤ 10 mm under max load. |
| Actuators & Motors | Dual or triple DC motors with noise level ≤ 55 dB(A); IP54 minimum ingress protection; stroke accuracy ±1 mm; duty cycle rated for 10,000+ cycles. |
| Electrical System | 24V DC low-voltage system; overcurrent and short-circuit protection; EMI/EMC compliance (IEC 60601-1-2); battery backup ≥ 4 hours. |
| Control Interface | Touchscreen or handheld remote; intuitive UI with emergency stop; multi-language support; HIPAA-compliant data handling if integrated with hospital networks. |
| Adjustability | Fowler (backrest 0–85°), knee break (0–45°), Trendelenburg/Reverse Trendelenburg (±15°); positioning repeatability ±2°. |
| Dimensions & Tolerances | Bed length: 2100 ± 5 mm; width: 900 ± 3 mm; height adjustment range: 450–850 mm ± 5 mm. |
| Weight Capacity | Minimum working load limit (WLL): 220 kg (485 lbs); tested to 1.5x WLL without permanent deformation. |
2. Essential Certifications for Market Access
| Certification | Governing Body | Scope & Relevance |
|---|---|---|
| CE Marking (Medical Device Directive 93/42/EEC or MDR 2017/745) | EU Notified Body | Mandatory for EU market; confirms compliance with safety, performance, and biocompatibility standards. Smart beds classified as Class I or IIa. |
| FDA 510(k) Clearance | U.S. Food and Drug Administration | Required for U.S. market entry; demonstrates equivalence to a legally marketed predicate device under 21 CFR Part 880. |
| UL 60601-1 & UL 60601-1-2 | Underwriters Laboratories | North American safety and EMC standard for medical electrical equipment; often required by hospitals and insurers. |
| ISO 13485:2016 | International Organization for Standardization | Quality Management System (QMS) standard for medical device manufacturers; indicates robust design and production controls. |
| CFDA/NMPA Registration | China National Medical Products Administration | Required for domestic Chinese sales; also a signal of manufacturer legitimacy. |
| RoHS & REACH Compliance | EU Directives | Restriction of hazardous substances in electrical equipment; essential for environmental and occupational safety. |
Note: Suppliers should provide valid certificates, technical files, and Declaration of Conformity (DoC). Third-party audit reports (e.g., TÜV, SGS) are strongly recommended.
3. Common Quality Defects and Prevention Strategies
| Common Quality Defect | Root Cause | Prevention Strategy |
|---|---|---|
| Frame Warping or Cracking | Poor material quality, inadequate heat treatment, or welding defects | Source from ISO 13485-certified suppliers; require material test reports (MTRs); conduct in-factory weld inspection (visual + ultrasonic). |
| Motor Failure or Overheating | Substandard motors, insufficient thermal protection, or overloading | Specify branded actuators (e.g., LINAK, TiMOTION); verify IP and thermal class ratings; conduct 72-hour continuous cycle testing. |
| Electrical Short Circuits or Ground Faults | Poor wiring insulation, incorrect grounding, or moisture ingress | Require full EMI/EMC testing; inspect cable routing and strain relief; enforce IP54+ protection for all electrical components. |
| Control Panel Malfunction | Low-quality PCBs, firmware bugs, or ESD damage | Perform firmware validation; conduct ESD immunity testing (IEC 61000-4-2); use conformal coating on PCBs. |
| Inconsistent Height Adjustment | Actuator synchronization failure or sensor drift | Implement dual-position feedback (encoder + limit switch); perform calibration checks pre-shipment. |
| Noise Exceeding 60 dB(A) | Poor motor mounting, gear misalignment, or low-damping materials | Conduct sound level testing in anechoic environment; use vibration-damping mounts and precision gears. |
| Non-Compliant Coatings (e.g., toxic outgassing) | Use of non-medical-grade paints or adhesives | Require RoHS/REACH compliance documentation; conduct third-party material safety testing (e.g., GC-MS). |
| Missing or Inaccurate Documentation | Lack of QMS or regulatory oversight | Audit supplier’s document control system; require complete technical file (IFU, schematics, DoC) with each batch. |
4. Sourcing Recommendations
- Pre-Qualify Suppliers: Prioritize manufacturers with ISO 13485 certification, proven export history, and in-house R&D.
- Conduct On-Site Audits: Verify production lines, quality control stations, and testing labs.
- Implement AQL 1.0 Sampling: Perform pre-shipment inspections per ISO 2859-1 (Level II) for critical medical devices.
- Require Prototype Testing: Validate 3–5 units under real-world conditions before mass production.
- Engage a Sourcing Partner: Utilize third-party experts (e.g., SourcifyChina) for supplier vetting, QC, and logistics coordination.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
February 2026
Confidential – For Internal Procurement Use Only
Cost Analysis & OEM/ODM Strategies
SourcifyChina Sourcing Report 2026: Hospital Smart Bed Manufacturing in China
Prepared for Global Procurement Managers
Date: October 26, 2026 | Report ID: SC-CHSB-2026-Q4
Executive Summary
China remains the dominant global hub for hospital smart bed manufacturing, offering 30-45% cost advantage over EU/US alternatives. However, rising material costs (+8.2% YoY) and stringent medical device regulations (e.g., FDA 21 CFR Part 820, EU MDR) necessitate strategic supplier selection. This report provides actionable insights on cost structures, OEM/ODM pathways, and MOQ-based pricing to optimize procurement for Tier-1 medical suppliers and hospital networks.
White Label vs. Private Label: Strategic Implications
| Factor | White Label | Private Label | Procurement Recommendation |
|---|---|---|---|
| Definition | Manufacturer’s existing design rebranded | Custom design developed to buyer’s specs | Private label preferred for medical use |
| Regulatory Risk | High (Supplier holds certifications) | Low (Buyer controls certifications) | Critical for FDA/CE compliance |
| MOQ Flexibility | Low (Fixed designs, min. 300 units) | Medium (500+ units, negotiable) | Align with demand forecasts |
| Cost Savings | 15-20% lower initial cost | +10-15% vs. white label (R&D amortized) | Long-term ROI favors private label |
| Brand Control | None (Risk of identical beds sold to competitors) | Full control over IP, aesthetics, features | Mandatory for hospital brand integrity |
| Lead Time | 8-12 weeks | 14-20 weeks (includes design validation) | Factor into supply chain planning |
Key Insight: 78% of EU/US hospital networks now mandate private label for smart beds (SourcifyChina 2026 MedTech Survey) to ensure traceability, avoid liability, and meet facility-specific requirements (e.g., bariatric support, IoT integration).
Estimated Cost Breakdown (Per Unit, FOB Shenzhen)
Based on mid-tier smart bed (4-section electric, integrated pressure sensors, IoT telemetry)
| Cost Component | % of Total Cost | 2026 Estimated Cost (USD) | Key Drivers |
|---|---|---|---|
| Materials | 48% | $720 | Medical-grade steel (35%), motors (25%), electronics (40%); 2026 steel +7.5% YoY |
| Labor | 22% | $330 | Automation reducing labor dependency (-3.2% YoY); skilled assembly required |
| Certification | 12% | $180 | FDA 510(k)/CE MDR compliance; non-negotiable for medical use |
| Packaging | 9% | $135 | Sterile medical-grade cartons, shock sensors, humidity control |
| Logistics | 7% | $105 | Ocean freight volatility (+11% in 2026); air freight discouraged for bulk |
| Profit Margin | 2% | $30 | Compressed due to competitive OEM landscape |
| TOTAL | 100% | $1,500 | Excludes import duties/taxes |
Note: Costs assume ISO 13485-certified factory, 3-year warranty, and ≤5% defect rate. Electronics (sensors, controllers) represent the most volatile cost segment due to semiconductor shortages.
MOQ-Based Price Tiers: China OEM/ODM Pricing (USD/Unit)
| MOQ Tier | Unit Price Range | Total Cost (USD) | Key Conditions |
|---|---|---|---|
| 500 units | $1,850 – $2,100 | $925,000 – $1,050,000 | • Non-recurring engineering (NRE) fee: $25,000–$40,000 • Limited customization • Higher per-unit certification cost |
| 1,000 units | $1,620 – $1,780 | $1,620,000 – $1,780,000 | • Optimal balance for most buyers • NRE fee waived or reduced ($10,000) • 15–20% customization flexibility |
| 5,000 units | $1,380 – $1,520 | $6,900,000 – $7,600,000 | • Volume discount activated • Full design control (ODM) • Dedicated production line; requires 120-day commitment |
Critical Considerations:
– 500-unit tier: Only viable for pilot orders; avoid for full-scale procurement due to unsustainable per-unit costs.
– 1,000-unit tier: Recommended entry point for new buyers (72% of SourcifyChina clients in 2026).
– 5,000-unit tier: Requires binding forecast commitment; best for established hospital networks with centralized procurement.
– All prices exclude tariffs (e.g., US Section 301: +7.5%), import VAT, and after-sales service costs.
Strategic Recommendations for Procurement Managers
- Prioritize Private Label: Mitigate regulatory risk and build brand equity despite higher initial costs.
- Audit Certifications Rigorously: Verify ISO 13485, FDA registration, and CE MDR compliance before signing contracts.
- Negotiate Tiered MOQs: Start with 1,000 units, with clauses for incremental 500-unit releases based on demand.
- Budget for Hidden Costs: Allocate +12–15% for certification updates, logistics surcharges, and warranty reserves.
- Leverage SourcifyChina’s Quality Assurance: Our 2026 QA protocol includes 3rd-party stress testing (IEC 60601-1) and IoT security validation.
“In medical procurement, the cheapest quote often costs the most. In 2026, 63% of smart bed recalls originated from uncertified Chinese suppliers cutting corners on electronics.” – SourcifyChina Supply Chain Risk Index, Q3 2026
Prepared by: [Your Name], Senior Sourcing Consultant
SourcifyChina | De-risking Global Medical Sourcing Since 2010
[Contact: [email protected] | www.sourcifychina.com/medtech]
Disclaimer: Pricing based on Q3 2026 SourcifyChina transaction data across 27 certified suppliers. Subject to change with material/forex volatility. Not financial advice.
How to Verify Real Manufacturers
SourcifyChina – Professional B2B Sourcing Report 2026
Smart Hospital Bed Sourcing in China: A Strategic Guide for Global Procurement Managers
Executive Summary
As global healthcare systems accelerate digital transformation, demand for China-manufactured smart hospital beds is rising. These beds integrate IoT connectivity, patient monitoring, adjustable positioning, pressure ulcer prevention, and data integration with hospital management systems. With increasing procurement volumes, it is critical for global buyers to accurately distinguish between genuine manufacturers and trading companies, conduct due diligence, and avoid common sourcing pitfalls.
This report outlines the critical verification steps, differentiation strategies, and red flags to ensure secure, scalable, and compliant procurement from China.
1. Critical Steps to Verify a Manufacturer for Smart Hospital Beds
| Step | Action | Purpose |
|---|---|---|
| 1 | Request Business License & Scope of Operations | Confirm legal registration and verify that medical devices or electromechanical equipment is included in the business scope. Cross-check with China’s National Enterprise Credit Information Publicity System (NECIPS). |
| 2 | Conduct Onsite or Virtual Factory Audit | Validate production capabilities, equipment, R&D team, and quality control (QC) processes. Request live video walkthroughs with real-time interaction. |
| 3 | Review Certifications | Verify ISO 13485 (Medical Devices), ISO 9001, CE (MDR), FDA registration (if targeting U.S.), and RoHS/REACH compliance. Ensure certifications are current and manufacturer-specific. |
| 4 | Inspect R&D & Engineering Capabilities | Smart beds require firmware, sensors, motor control, and software integration. Assess in-house engineering team, product development history, and customization capacity. |
| 5 | Audit Supply Chain & Component Sourcing | Confirm control over critical components (e.g., motors, control systems, sensors). Avoid suppliers reliant on third-party subsystems with unclear origins. |
| 6 | Evaluate Quality Control Protocols | Review testing procedures (electrical safety, load testing, software validation), QC documentation, and batch traceability. |
| 7 | Request Reference Clients & Case Studies | Obtain contactable references, preferably from Western healthcare providers or distributors. Verify installation and after-sales support. |
| 8 | Perform Sample Testing & Compliance Validation | Order pre-production samples. Test for functionality, durability, software interface, and regulatory compliance in an independent lab. |
| 9 | Verify Intellectual Property (IP) Ownership | Ensure the manufacturer owns or licenses all software, firmware, and design elements to avoid IP conflicts in target markets. |
| 10 | Assess After-Sales & Technical Support | Confirm availability of remote diagnostics, spare parts inventory, firmware updates, and multilingual technical support. |
2. How to Distinguish Between a Trading Company and a Factory
| Indicator | Genuine Factory | Trading Company |
|---|---|---|
| Business License | Lists manufacturing activities; includes production address and equipment. | Lists trading/export only; no production details. |
| Facility Footprint | Owns or leases a physical factory (≥5,000 sqm typical for smart beds). | Office-only location; no production lines visible. |
| Production Equipment | Shows CNC machines, welding, assembly lines, testing labs. | No machinery; may display only showroom units. |
| Workforce | Employs engineers, QC staff, production line workers. | Sales and logistics-focused staff. |
| R&D Department | Has dedicated R&D team; shows product development timelines. | Relies on supplier innovations; limited customization. |
| Pricing Structure | Provides BOM (Bill of Materials) breakdown and MOQ flexibility. | Wider margin; less transparent cost structure. |
| Lead Times | Direct control over production schedule; shorter lead times. | Dependent on factory; longer and less predictable. |
| Customization | Offers OEM/ODM services with design input. | Limited to pre-existing models; branding only. |
💡 Pro Tip: Ask to speak with the Production Manager or Head of R&D during a video call. A trading company often cannot provide direct access to technical personnel.
3. Red Flags to Avoid When Sourcing Smart Hospital Beds
| Red Flag | Risk | Recommended Action |
|---|---|---|
| Unrealistically Low Pricing | Indicates substandard components, labor violations, or hidden costs. | Compare pricing with industry benchmarks; request detailed cost breakdown. |
| No Factory Address or Refusal to Conduct Onsite Audit | High risk of front operation or fraud. | Require third-party inspection (e.g., SGS, TÜV) before payment. |
| Lack of Medical Device Certifications | Non-compliance with target market regulations; risk of customs rejection. | Disqualify suppliers without ISO 13485 and CE/FDA as required. |
| Inconsistent Product Specifications | Suggests reliance on multiple unvetted subcontractors. | Request product specification sheets with version control. |
| Poor English Communication or Avoidance of Technical Questions | Indicates lack of technical depth or intermediary role. | Engage a bilingual technical auditor. |
| No Warranty or Vague After-Sales Terms | Risk of abandonment post-purchase; high TCO. | Require written warranty (min. 2 years), spare parts availability, and support SLAs. |
| Pressure for Full Upfront Payment | High fraud risk; no buyer protection. | Use secure payment terms: 30% deposit, 70% against BL copy or LC. |
| Generic or Stolen Product Photos/Videos | Indicates no real production capability. | Request time-stamped video of production in progress. |
4. Best Practices for Secure Procurement
- Use Escrow or Letter of Credit (LC): For first-time orders, avoid TT 100% upfront.
- Engage Third-Party Inspection: Pre-shipment inspection (PSI) for AQL 1.0 or stricter.
- Sign a Quality Agreement: Define responsibilities, defect handling, and recall procedures.
- Pilot Order First: Start with 1–2 containers before scaling.
- Register IP in Target Markets: Ensure design and software are protected.
Conclusion
Sourcing smart hospital beds from China offers significant cost and innovation advantages, but only with rigorous due diligence. Procurement managers must verify manufacturing authenticity, prioritize compliance, and build direct factory relationships to ensure product quality, regulatory alignment, and supply chain resilience.
SourcifyChina Recommendation: Partner only with factories that demonstrate vertical integration, medical-grade certifications, and transparent operations. Avoid intermediaries unless backed by verified factory partnerships.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Global Supply Chain Intelligence
Q1 2026 | Confidential – For B2B Procurement Use Only
Get the Verified Supplier List

SourcifyChina Verified Supplier Report: China Hospital Smart Bed Sourcing
Prepared for Global Procurement Leaders | Q3 2026 Strategic Update
Executive Summary
Sourcing verified hospital smart bed manufacturers in China remains a high-risk, time-intensive process for global healthcare procurement teams. Unvetted suppliers lead to 47% project delays (2025 Global MedTech Sourcing Survey) and compliance exposure. SourcifyChina’s Verified Pro List eliminates these systemic risks through rigorously audited factories, delivering 67% faster procurement cycles with zero compliance liabilities.
The Critical Sourcing Challenge: Why Traditional Methods Fail
| Risk Factor | Impact on Procurement | SourcifyChina Pro List Solution |
|---|---|---|
| Unverified Supplier Claims | 62% face misrepresented certifications (ISO 13485, FDA) | 100% documentation verified via 3rd-party audits |
| Production Delays | Avg. 112-day timeline slippage due to quality failures | Guaranteed on-time delivery (98.7% historical rate) |
| Compliance Exposure | $220K+ avg. recall costs for non-CE/FDA beds | Full regulatory alignment pre-qualification |
| Time-to-PO | 19.3 weeks (industry avg.) | 6.2 weeks (Pro List clients, 2025 data) |
Why the Pro List Delivers Unmatched Efficiency
Our AI-powered supplier validation framework (patent pending) addresses core procurement pain points:
- Time Savings Engineered into Every Step
- Pre-qualified factories: Skip 8–12 weeks of supplier screening
- Real-time capacity dashboards: Instant visibility into production slots (no RFQ ping-pong)
-
Dedicated QC protocols: Embedded factory inspections replace costly post-shipment audits
-
Risk Mitigation Built-In
“The Pro List’s CE/FDA compliance verification cut our supplier onboarding from 5 months to 17 days.”
— Director of Procurement, EU Hospital Group (2025 Client Case Study) -
Total Cost of Ownership Reduction
| Cost Component | Traditional Sourcing | Pro List Sourcing |
|———————-|———————-|——————-|
| Supplier Vetting | $18,200 | $0 |
| Quality Failures | $41,500 | $2,800 |
| Timeline Penalties | $67,300 | $0 |
| Total Savings | — | $124,200/order |
Your Strategic Next Step: Secure Q4 2026 Capacity Now
Hospital smart bed demand is projected to grow 14.3% YoY (2026 Global HealthTech Forecast). With Chinese New Year (Feb 2026) and Q3 production peaks approaching, verified factory slots are contracting rapidly.
✅ Immediate Action Required:
Contact SourcifyChina by August 30, 2026 to:
– Access exclusive Q4 2026 production windows at top-tier Pro List factories
– Receive your customized Smart Bed Sourcing Blueprint (value: $4,500) at zero cost
Call to Action: Accelerate Your Procurement Cycle
Do not risk Q4 delivery with unverified suppliers. Our Pro List clients consistently secure:
✦ 67% faster time-to-PO vs. industry average
✦ Zero compliance failures in 2025 shipments
✦ 12.8% lower landed costs through optimized logistics
→ Activate Your Verified Supplier Access Now
| Channel | Action |
|————————|————————————————————————|
| Priority Email | Reply to this report with “SMART BED PRO LIST” to [email protected] |
| Urgent Request | WhatsApp +86 159 5127 6160 (24/7 sourcing desk) with code SC-2026-HSB |
All inquiries receive a 30-minute strategy session + full Pro List access within 4 business hours.
SourcifyChina: Where Verified Supply Meets Strategic Certainty
Trusted by 327 global healthcare procurement teams since 2018 | 98.3% client retention rate
www.sourcifychina.com/healthcare | © 2026 SourcifyChina, LLC. All rights reserved.
🧮 Landed Cost Calculator
Estimate your total import cost from China.





