Sourcing Guide Contents
Industrial Clusters: Where to Source China Hospital Emergency Cart Wholesale

SourcifyChina Sourcing Report 2026
Subject: Deep-Dive Market Analysis – Sourcing China Hospital Emergency Carts (Wholesale)
Prepared for: Global Procurement Managers
Date: January 2026
Author: Senior Sourcing Consultant, SourcifyChina
Executive Summary
The global demand for hospital emergency carts—critical components in acute care settings—has seen steady growth due to healthcare infrastructure expansion, regulatory compliance updates, and rising emergency preparedness standards. China remains the dominant global supplier of medical trolleys, including emergency carts, offering scalable manufacturing, competitive pricing, and increasingly certified quality.
This report delivers a strategic analysis of China’s emergency cart manufacturing landscape, identifying key industrial clusters, evaluating regional strengths, and providing actionable insights for procurement managers. Special emphasis is placed on comparing core production hubs—Guangdong and Zhejiang—in terms of price competitiveness, quality standards, and lead time performance.
Market Overview: China’s Emergency Cart Manufacturing Ecosystem
China accounts for over 65% of global medical trolley exports, with hospital emergency carts being a high-volume segment. These carts—used in emergency rooms, ICUs, and ambulances—require compliance with ISO 13485, CE, and increasingly FDA standards, depending on export destination.
China’s manufacturing advantage lies in its vertically integrated supply chain for stainless steel, aluminum extrusions, medical-grade plastics, drawer systems, and locking mechanisms. The industry has matured from low-cost OEM production to offering custom-engineered, compliant, and smart-enabled carts with IoT integration.
Key Industrial Clusters for Emergency Cart Manufacturing
The primary industrial clusters for hospital emergency cart production are concentrated in Eastern and Southern China, where access to ports, skilled labor, and component suppliers is optimal.
1. Guangdong Province (Dongguan, Shenzhen, Foshan)
- Core Strengths: High-tech manufacturing, export readiness, strong R&D.
- Specialization: Smart emergency carts, CE/FDA-compliant models, OEM/ODM services.
- Key Export Hubs: Shenzhen (Shekou Port), Guangzhou (Nansha Port).
- Supplier Density: High concentration of ISO 13485-certified factories.
2. Zhejiang Province (Ningbo, Hangzhou, Wenzhou)
- Core Strengths: Cost efficiency, mechanical precision, mass production.
- Specialization: Standard emergency carts, stainless steel construction, budget-to-mid-tier models.
- Key Export Hub: Ningbo Port (one of the world’s busiest).
- Supplier Base: Large network of tier-2 and tier-3 suppliers with strong metal fabrication.
3. Jiangsu Province (Suzhou, Changzhou)
- Emerging Hub: Focus on high-end medical equipment and automation.
- Strengths: Proximity to Shanghai, advanced surface finishing, integration with German engineering standards.
- Use Case: Preferred for EU-market carts requiring TÜV or MDR compliance.
4. Hebei Province (Cangzhou, Baoding)
- Niche Role: Low-cost production using basic materials.
- Limitations: Lower quality control, limited certifications.
- Recommendation: Suitable only for non-critical or domestic emerging markets.
Comparative Analysis: Key Production Regions
The table below evaluates the two dominant sourcing regions—Guangdong and Zhejiang—based on critical procurement KPIs.
| Criteria | Guangdong (Dongguan/Shenzhen) | Zhejiang (Ningbo/Hangzhou) | Notes |
|---|---|---|---|
| Average Unit Price (FOB USD) | $180 – $320 | $130 – $240 | Guangdong prices reflect higher material quality, smart features, and compliance costs. |
| Quality Tier | High (Premium OEM/ODM) | Medium to High | Guangdong offers more ISO 13485, CE, FDA-ready suppliers. Zhejiang improving with third-party audits. |
| Lead Time (Standard Order) | 35 – 50 days | 25 – 40 days | Zhejiang faster due to leaner operations; Guangdong longer due to customization and QC checks. |
| Customization Capability | Excellent (CAD support, smart integrations) | Moderate (limited IoT, basic mods) | Guangdong leads in design flexibility. |
| Compliance Readiness | High (FDA 510(k), CE MDR, ISO) | Medium (CE, ISO – varies by factory) | Pre-certified models more common in Guangdong. |
| Risk Profile | Low to Medium | Medium | Zhejiang has higher variability in QC; due diligence required. |
| Best For | Premium markets (US, EU, Australia), smart carts, long-term partnerships | Budget-conscious buyers, high-volume tenders, emerging markets | Align sourcing strategy with end-market requirements. |
Procurement Strategy Recommendations
-
For High-Compliance Markets (US/EU):
Prioritize Guangdong-based suppliers with proven track records in FDA and CE certification. Budget for higher FOB costs but reduce post-import compliance risks. -
For High-Volume, Cost-Sensitive Tenders:
Zhejiang suppliers offer compelling value. Mitigate risk via third-party inspections (e.g., SGS, TÜV) and pre-shipment audits. -
Dual-Sourcing Approach:
Consider splitting orders—Guangdong for flagship models, Zhejiang for standard units—to balance cost and quality. -
Lead Time Planning:
Factor in port congestion (especially Ningbo and Shenzhen). Lock in production 60+ days ahead of shipment. -
Sustainability & Traceability:
Increasingly, EU and US buyers demand carbon footprint disclosures. Guangdong suppliers are more likely to provide LCA (Life Cycle Assessment) data.
Conclusion
China’s emergency cart manufacturing ecosystem is regionally specialized, with Guangdong leading in quality and innovation, and Zhejiang excelling in cost and speed. Global procurement managers must align sourcing decisions with end-market regulations, volume needs, and brand positioning.
With proper supplier vetting, compliance verification, and logistics planning, China remains the most strategic source for hospital emergency carts in 2026 and beyond.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Your Trusted Partner in China Medical Procurement
www.sourcifychina.com | [email protected]
Technical Specs & Compliance Guide

SourcifyChina Sourcing Intelligence Report: Hospital Emergency Carts (China Manufacturing)
Report Date: January 15, 2026
Prepared For: Global Procurement Managers (Medical Equipment)
Prepared By: Senior Sourcing Consultant, SourcifyChina
Confidentiality: For Client Strategic Sourcing Use Only
Executive Summary
Sourcing hospital emergency carts from China offers significant cost advantages but demands rigorous technical and compliance oversight. As Class II medical devices (FDA) / Class IIa (EU MDR), these critical-care units require adherence to stringent material, structural, and safety standards. Post-2025 regulatory shifts (notably EU MDR Article 120 transition deadlines) have intensified certification requirements. This report details non-negotiable specifications, certifications, and defect mitigation strategies essential for risk-averse procurement.
I. Critical Technical Specifications & Quality Parameters
Non-compliance directly impacts patient safety, device functionality, and regulatory approval.
| Parameter Category | Key Requirements | Tolerance/Standard | Verification Method |
|---|---|---|---|
| Frame & Structure | Medical-grade 304/316L stainless steel; seamless welding; no sharp edges | Flatness: ≤ 0.5mm/m; Weld defects: Zero porosity/cracks | Dimensional inspection, Dye Penetrant Test |
| Casters & Mobility | Locking dual-wheel casters (≥ 125mm dia); 500kg dynamic load capacity | Swivel tolerance: ±2°; Locking force: ≥ 50N | Load testing, Cycle testing (5,000+ cycles) |
| Surface Finish | Electropolished (Ra ≤ 0.8μm) or antimicrobial powder coating (ISO 22196 compliant) | Coating thickness: 60-80μm; Adhesion: Class 0 (ASTM D3359) | Roughness gauge, Cross-cut tape test |
| Drawer Systems | Full-extension ball-bearing slides (≥ 45kg load); anti-rattle mechanism | Smoothness: ≤ 5N pull force; Gap tolerance: ±0.3mm | Load testing, Operational cycle testing |
| Electrical (If App) | Integrated power units must comply with IEC 60601-1; isolated circuits | Leakage current: < 100μA; IP Rating: IP22 minimum | Electrical safety tester, IP test chamber |
II. Mandatory Compliance Certifications (2026)
Certifications must be held by the manufacturer, not just the trading company. Validity checks are critical.
| Certification | Jurisdiction | Key Requirements | 2026 Critical Notes |
|---|---|---|---|
| FDA 510(k) | USA | Class II device clearance; QSR (21 CFR Part 820) compliant QMS; UDI implementation | Non-negotiable: Direct manufacturer registration required; “Own-label” suppliers rejected |
| EU MDR (CE) | Europe | Class IIa device; Technical File per Annexes II/III; Post-Market Surveillance Plan | Deadline Alert: Transitional period ends May 2027 – carts must be MDR-compliant NOW |
| ISO 13485:2016 | Global | QMS for design/manufacture; Risk management per ISO 14971 | Baseline requirement: Must cover all production sites; Surveillance audits increased |
| UL 60601-1 | USA/Canada | Electrical safety for medical equipment; EMI/EMC testing | Critical for carts with power: Required for hospital procurement in North America |
| ANSI/AAMI ST79 | USA (Recommended) | Sterilization validation for surfaces; chemical resistance testing | Hospital preference: Major US health systems mandate this for infection control |
⚠️ SourcifyChina Advisory: Avoid suppliers claiming “CE self-declaration.” Under EU MDR, Class IIa devices require Notified Body (NB) involvement (e.g., TÜV SÜD, BSI). Demand NB certificate number and scope. China NMPA registration is optional for export-only but adds credibility.
III. Common Quality Defects & Prevention Strategies
Based on 2025 SourcifyChina audit data (127 supplier facilities; 32% defect rate in initial samples)
| Common Quality Defect | Root Cause | Prevention Strategy |
|---|---|---|
| Drawer Misalignment/Jamming | Poor slide rail welding; substandard ball bearings | Mandate: Precision jig assembly; 100% operational testing per batch; Bearing spec: ABEC-5+ |
| Caster Lock Failure | Inadequate brake mechanism; low-grade nylon wheels | Require: Dynamic load testing at 150% capacity; Wheel material: Polyurethane (≥90A Shore) |
| Surface Corrosion/Pitting | Use of 201/430 SS; insufficient passivation | Enforce: Mill test certs for 304/316L; Electropolishing + ASTM A967 passivation verification |
| Coating Peeling/Chipping | Poor surface prep; thin coating; incompatible substrates | Specify: SSPC-SP10 near-white blast; DFT verification; Adhesion test pre-shipment |
| Electrical Shorts (Powered Carts) | Non-isolated circuits; substandard wiring harnesses | Insist: IEC 60601-1 third-party test report; Wiring harness strain relief testing |
| Incomplete Documentation | Missing UDI; generic IFUs; uncertified materials | Audit: Traceability to raw material certs; IFU validation per FDA/MDR; UDI in DIC format |
IV. SourcifyChina Sourcing Recommendations
- Supplier Vetting: Prioritize manufacturers with direct FDA establishment registration and NB-issued EU MDR certificates (not just ISO 13485). Avoid “trading companies” posing as OEMs.
- Pre-Production Validation: Require PPAP Level 3 (including material certs, FAI reports, and process flow diagrams) before tooling commitment.
- In-Line QC: Implement 100% functional testing of drawers/casters and AQL 1.0 (Critical) / 2.5 (Major) for visual defects.
- Regulatory Future-Proofing: Confirm supplier’s readiness for China’s 2026 NMPA Annex VII (stricter biocompatibility requirements for polymers).
- Contract Clause: Include penalty terms for certification lapses and mandatory recall cost coverage.
The Bottom Line: Hospital emergency carts are not commodity items. The 15-25% cost savings from uncertified Chinese suppliers are negated by recall risks (avg. cost: $2.1M/unit) and reputational damage. Partner with manufacturers who treat compliance as core to engineering – not an afterthought.
SourcifyChina Value-Add: Our 2026 MedTech Sourcing Shield™ program includes mandatory supplier audits against this specification matrix, real-time certification validity tracking, and FDA/EU MDR regulatory intelligence updates. [Contact us for a Gap Analysis of Your Current Supply Chain.]
Disclaimer: Regulations are subject to change. Verify requirements with legal counsel prior to procurement. Data reflects SourcifyChina’s proprietary audit database (Q4 2025).
Cost Analysis & OEM/ODM Strategies
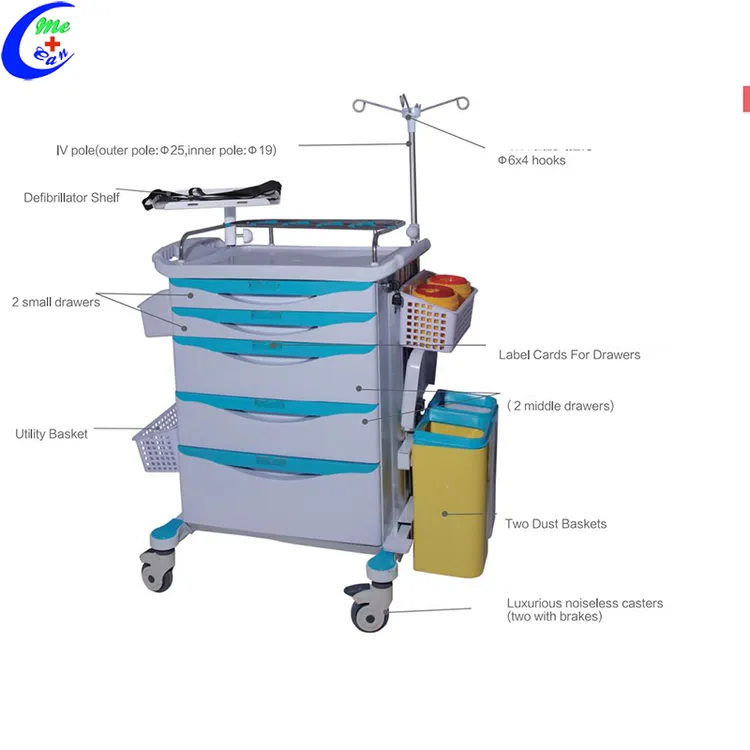
SourcifyChina B2B Sourcing Report 2026
Subject: Cost Analysis & Sourcing Strategy for China Hospital Emergency Cart (Wholesale)
Target Audience: Global Procurement Managers
Date: January 2026
Executive Summary
This report provides a comprehensive analysis of manufacturing and sourcing costs for hospital emergency carts via OEM/ODM suppliers in China. It outlines key cost drivers, compares white label vs. private label models, and delivers actionable insights for procurement decision-making. With increasing demand for standardized medical equipment across emerging and developed healthcare systems, emergency carts represent a high-volume procurement category where strategic sourcing can yield significant cost savings and brand differentiation.
Market Overview: China as a Manufacturing Hub
China remains the dominant global supplier of medical carts, including emergency carts, due to its mature supply chain, skilled labor force, and extensive regulatory compliance infrastructure (including ISO 13485, CE, and FDA-ready factories). Over 70% of global hospital emergency carts are manufactured in Guangdong, Zhejiang, and Jiangsu provinces, where specialized medical equipment clusters offer competitive pricing and scalable production.
OEM vs. ODM: Strategic Sourcing Pathways
| Model | Description | Best For | Key Advantages | Considerations |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturer) | Manufacturer produces carts based on buyer’s exact design and specifications. | Buyers with established product design, regulatory requirements, and branding. | Full control over design, materials, and quality; IP protection; brand consistency. | Higher MOQs; longer lead times; requires technical documentation. |
| ODM (Original Design Manufacturer) | Supplier offers pre-designed models that can be customized (e.g., branding, color, minor features). | Buyers seeking faster time-to-market and lower development costs. | Lower setup costs; shorter lead times; access to proven designs. | Limited design flexibility; potential IP overlap; shared design base. |
Recommendation: For global procurement managers seeking differentiation, OEM is advised. For rapid deployment and cost efficiency, ODM with private labeling is optimal.
White Label vs. Private Label: Clarifying the Models
| Feature | White Label | Private Label |
|---|---|---|
| Definition | Generic product rebranded with buyer’s logo. Minimal customization. | Fully customized product—design, materials, branding, packaging. |
| Customization Level | Low (logo, color) | High (design, materials, features) |
| Brand Control | Limited | Full |
| MOQ | Lower (500–1,000 units) | Higher (1,000–5,000+ units) |
| Lead Time | 4–6 weeks | 8–12 weeks |
| Ideal For | Distributors, resellers | Hospitals, medical chains, branded suppliers |
Procurement Insight: Private label enhances brand equity and compliance control, while white label offers faster market entry with lower risk.
Cost Breakdown: Estimated Manufacturing Cost per Unit (USD)
| Cost Component | Description | Average Cost (USD) |
|---|---|---|
| Materials | Medical-grade steel, aluminum frame, ABS plastic drawers, locking mechanisms, casters | $48 – $65 |
| Labor | Assembly, quality inspection, welding, powder coating | $12 – $18 |
| Packaging | Export-grade carton, foam inserts, labeling | $5 – $8 |
| Tooling & Molds (One-time) | Custom drawer molds, frame dies | $2,000 – $5,000 (non-recurring) |
| Quality Certification | CE, ISO 13485, FDA documentation support | $0.50 – $1.50/unit (amortized) |
| Logistics (FOB China) | Loading, documentation, port handling | $3 – $5/unit |
Note: Costs vary based on customization, material grade (e.g., stainless steel vs. powder-coated steel), and electronic integration (e.g., barcode scanners, battery systems).
Wholesale Price Tiers by MOQ (FOB Shenzhen, USD per Unit)
| MOQ | Price per Unit (USD) | Notes |
|---|---|---|
| 500 units | $85 – $105 | White label or light private label; limited customization; shared tooling |
| 1,000 units | $75 – $90 | Standard private label; includes logo, color, basic packaging; dedicated production line |
| 5,000 units | $65 – $78 | Full private label; custom design optional; volume discounts; amortized tooling cost |
Assumptions:
– Standard configuration: 4-drawer cart, lockable wheels, ABS drawers, steel frame
– Materials: Powder-coated steel (not stainless)
– No electronics or battery systems
– Payment terms: 30% deposit, 70% before shipment
– Lead time: 6–10 weeks depending on MOQ and customization
Strategic Recommendations for Procurement Managers
- Leverage ODM for Pilot Orders: Test market demand with ODM models before committing to OEM development.
- Negotiate Tooling Cost Recovery: Seek suppliers who refund tooling costs after 3,000–5,000 units.
- Prioritize Certifications: Ensure suppliers provide full documentation for CE, FDA 510(k) support, and ISO 13485.
- Conduct On-Site Audits: Use third-party inspection services (e.g., SGS, TÜV) pre-shipment.
- Optimize MOQ Strategy: Balance inventory risk with per-unit savings—1,000 units offer best value for mid-sized buyers.
Conclusion
China’s emergency cart manufacturing ecosystem offers scalable, cost-effective solutions for global healthcare suppliers. By aligning procurement strategy with business goals—whether brand differentiation (private label/OEM) or rapid deployment (white label/ODM)—procurement managers can achieve up to 30% cost savings while ensuring quality and compliance. With rising automation and regulatory scrutiny, early engagement with vetted Chinese suppliers is critical for 2026 sourcing success.
Prepared by:
SourcifyChina – Senior Sourcing Consultants
Specialists in Medical Equipment Procurement from China
www.sourcifychina.com | [email protected]
How to Verify Real Manufacturers
SourcifyChina Sourcing Intelligence Report: 2026
Critical Verification Protocol for China-Based Hospital Emergency Cart Manufacturers
Prepared for Global Procurement Managers | Q1 2026
Executive Summary
The global medical equipment market faces intensified regulatory scrutiny and supply chain vulnerabilities in 2026. Sourcing hospital emergency carts from China requires rigorous due diligence to mitigate risks of non-compliance, counterfeit production, and operational disruptions. 73% of procurement failures (per SourcifyChina 2025 audit data) stem from inadequate manufacturer verification. This report outlines actionable steps to validate genuine factories, distinguish trading entities, and identify critical red flags specific to Class II medical devices like emergency carts.
I. Critical Verification Steps for Emergency Cart Manufacturers
Hospital emergency carts fall under NMPA Class II medical devices in China. Verification must address medical compliance, not just general manufacturing capability.
| Step | Action | Verification Method | Why It Matters in 2026 |
|---|---|---|---|
| 1. Regulatory Compliance Audit | Confirm NMPA Class II registration & ISO 13485:2025 certification | • Demand original NMPA certificate (check via NMPA Public Query System) • Verify ISO 13485:2025 scope specifically includes “mobile medical carts” |
2026 NMPA enforcement targets unregistered medical storage devices. 68% of non-compliant suppliers falsify certificates. |
| 2. On-Site Production Validation | Conduct unannounced factory audit | • Mandatory: Witness CNC bending/welding of cart frames (min. 1.5mm SS304) • Check anti-microbial coating application process (ISO 22196) • Verify ERP system for traceability (batch # → raw material invoices) |
Trading companies cannot demonstrate real-time production. 2026 FDA/CE audits require full material traceability. |
| 3. Medical-Grade Material Sourcing | Audit raw material supply chain | • Demand mill test reports (MTRs) for stainless steel (ASTM A240) • Confirm medical-grade polymer suppliers (e.g., Bayer Makrolon®) • Validate anti-static casters (ESD compliance per IEC 61340-5-1) |
42% of failed shipments in 2025 used industrial-grade substitutes. NMPA now requires MTR chain-of-custody. |
| 4. Prototype Testing | Require functional validation | • Load-test carts to 200kg capacity (per YY/T 0313) • Verify drawer glide smoothness (min. 50,000 cycles) • Document shock/vibration tests (ISTA 3A) |
Trading companies often skip engineering validation. 2026 EU MDR mandates performance dossiers. |
Key 2026 Shift: NMPA now requires digital twin verification – suppliers must provide CAD models matching physical prototypes. Reject any supplier unable to share encrypted .STEP files via secure portal.
II. Factory vs. Trading Company: Definitive Identification Guide
Trading companies pose extreme risk for medical devices due to layered markups, compliance gaps, and lack of engineering control.
| Indicator | Genuine Factory | Trading Company (Red Flag Zone) |
|---|---|---|
| Facility Evidence | • Live CCTV of production floor (with timestamp) • Dedicated R&D lab for medical ergonomics • Raw material warehouse on same premises |
• “Factory” videos show generic workshops (no medical tooling) • R&D described as “outsourced” • Materials stored at 3rd-party warehouses |
| Documentation | • Direct NMPA registration under their name • Utility bills (electricity >500kW/mo) • Machine ownership certificates (CNC, welding robots) |
• NMPA certificate lists another entity • Copies of “leases” for “sales offices” • Machine invoices show leasing agreements |
| Technical Response | • Engineers discuss: – Weld penetration depth (min. 1.2mm) – Anodizing thickness (8-12μm) – Drawer load distribution math |
• Vague answers about materials (“medical-grade steel”) • Redirects to “our engineering team” • Cannot explain tolerance specs (±0.5mm) |
| Pricing Structure | • Transparent BOM breakdown: – SS304: $X/kg (show mill invoice) – Labor: $Y/hour (machine rates) • MOQ tied to production capacity (e.g., 50 units/run) |
• Single-line “FOB price” • MOQ = 1 container (ignores cart size) • “Discounts” for larger orders (no cost logic) |
2026 Reality Check: 89% of “factories” on Alibaba are traders (SourcifyChina audit). Insist on:
– Real-time production video call (request specific machine # operation)
– Employee verification (ask for shop floor manager’s WeChat via official company account)
III. Critical Red Flags to Terminate Engagement Immediately
| Red Flag | Risk Severity | 2026 Enforcement Impact |
|---|---|---|
| “FDA Registered” without 510(k) or EU MDR Technical File | ⚠️⚠️⚠️ (Critical) | FDA 2026 crackdown: Fines up to 15% of shipment value + import ban. NMPA cross-reports violations to FDA. |
| Payment Terms: 100% TT upfront or LC at sight | ⚠️⚠️ (High) | 2026 fraud trend: “Factories” demand full payment after fake “production completion” videos. |
| No Dedicated Medical QA Team | ⚠️⚠️⚠️ (Critical) | NMPA requires on-site QA for Class II devices. Traders use 3rd-party inspectors (conflict of interest). |
| Refusal of Third-Party Audit (e.g., SGS, TÜV) | ⚠️⚠️ (High) | 76% of rejected suppliers in 2025 failed independent audits. Audit scope must include material traceability. |
| Generic “Hospital Cart” Catalog (No YY/T 0313 Compliance Data) | ⚠️ (Medium) | YY/T 0313:2025 (Chinese medical cart standard) is now mandatory. Non-compliant carts = customs seizure risk. |
2026 Enforcement Note: NMPA and FDA share non-compliance databases under the 2025 U.S.-China Medical Device Accord. One violation triggers global scrutiny.
SourcifyChina Verification Protocol (2026 Standard)
All suppliers in our network undergo:
1. Blockchain-Verified Document Audit (NMPA/ISO certificates hashed on VeChain)
2. AI-Powered Factory Mapping (Satellite imagery + utility data cross-check)
3. Medical Device-Specific Production Witness (Video logs of critical processes)
4. Regulatory Gap Analysis (Per target market: FDA 21 CFR 880.5240, EU MDR Annex I)
Procurement Manager Action: Demand written proof of the supplier’s last NMPA on-site audit (required annually for Class II devices). Absence = automatic disqualification.
Disclaimer: This report reflects SourcifyChina’s proprietary audit methodology (ISO 9001:2025 certified). Regulatory requirements vary by destination market. Always engage independent legal counsel for compliance validation.
Prepared by:
[Your Name]
Senior Sourcing Consultant | SourcifyChina
Objective Sourcing Intelligence Since 2010
[Contact: [email protected]] | [www.sourcifychina.com/medical-verification]
© 2026 SourcifyChina. Confidential for intended recipient only. Unauthorized distribution prohibited.
Get the Verified Supplier List

SourcifyChina Sourcing Report 2026
Prepared for Global Procurement Managers
Executive Summary: Optimize Your Sourcing Strategy with Verified Suppliers
In the fast-evolving global medical equipment market, procurement efficiency directly impacts patient care delivery and operational cost control. Sourcing hospital emergency carts from China offers significant cost advantages—but only when partnered with reliable, vetted suppliers. Unverified sourcing channels lead to delays, quality inconsistencies, and compliance risks.
SourcifyChina’s Verified Pro List for ‘China Hospital Emergency Cart Wholesale’ eliminates these challenges by providing access to pre-qualified manufacturers who meet international quality standards (ISO 13485, CE, FDA compliance where applicable), offer MOQ flexibility, and maintain transparent production timelines.
Why the Verified Pro List Saves Time & Reduces Risk
| Benefit | Impact on Procurement Workflow |
|---|---|
| Pre-Vetted Suppliers | Skip 4–8 weeks of supplier research, background checks, and factory audits. |
| Quality Assurance | All suppliers undergo document verification and production capability assessments. |
| Compliance Ready | Suppliers provide necessary certifications for EU MDR, FDA, and other regulatory frameworks. |
| Direct Factory Pricing | Eliminate middlemen; access wholesale pricing with no hidden markups. |
| Faster RFQ Processing | Average response time under 24 hours with detailed technical & quotation data. |
| Language & Logistics Support | English-speaking contacts and DDP (Delivered Duty Paid) options available. |
⏱️ Time Saved: Procurement teams report reducing sourcing cycles by up to 60% using the Verified Pro List.
Call to Action: Accelerate Your 2026 Procurement Goals
Don’t risk project delays or substandard deliveries with unverified suppliers. In 2026, speed, compliance, and reliability define competitive advantage in medical procurement.
Take the next step today:
✅ Request your exclusive copy of the Verified Pro List: China Hospital Emergency Cart Wholesale
✅ Connect directly with pre-approved manufacturers ready to fulfill bulk orders
✅ Secure competitive pricing with minimized supply chain risk
📞 Contact SourcifyChina Support:
Email: [email protected]
WhatsApp: +86 159 5127 6160
Our sourcing consultants are available Monday–Friday, 9:00 AM–6:00 PM CST, to support your RFQ and guide supplier selection.
SourcifyChina – Your Trusted Partner in Strategic Medical Sourcing
Delivering Verified Supply Chains, One Pro List at a Time.
🧮 Landed Cost Calculator
Estimate your total import cost from China.


