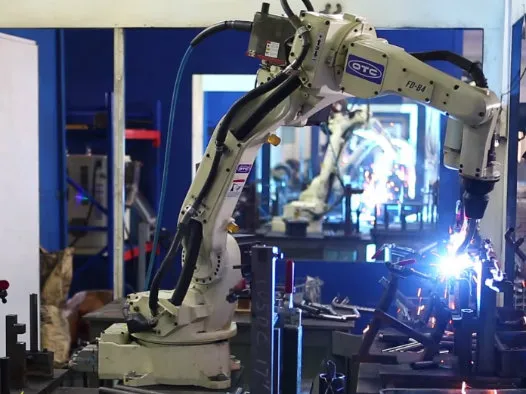
Sourcing Guide Contents
Industrial Clusters: Where to Source China Heavy Duty Transfer Chair Company

Professional B2B Sourcing Report 2026
SourcifyChina | Global Sourcing Intelligence Division
Title: Deep-Dive Market Analysis – Sourcing Heavy-Duty Transfer Chairs from China
Prepared for: Global Procurement Managers
Date: Q1 2026
Executive Summary
The global demand for heavy-duty transfer chairs—critical mobility aids used in healthcare, rehabilitation centers, and elderly care facilities—has seen steady growth due to aging populations and increased focus on patient safety. China remains the dominant manufacturing hub for medical mobility equipment, offering competitive pricing, scalable production, and evolving technical capabilities.
This report provides a strategic sourcing analysis for heavy-duty transfer chairs manufactured in China, with a focus on identifying key industrial clusters, evaluating regional production strengths, and delivering actionable insights for procurement professionals.
Note: The term “China heavy duty transfer chair company” is interpreted as manufacturers in China producing heavy-duty transfer chairs (also known as bariatric transfer chairs, sit-to-stand chairs, or patient transfer aids).
Key Industrial Clusters for Heavy-Duty Transfer Chair Manufacturing
China’s medical mobility device manufacturing is concentrated in three primary industrial clusters, each offering distinct advantages in cost, quality, and supply chain efficiency:
| Province | Key Cities | Industrial Focus | Key Advantages |
|---|---|---|---|
| Guangdong | Shenzhen, Dongguan, Guangzhou | High-tech medical devices, OEM/ODM manufacturing | Proximity to ports, strong R&D, high automation |
| Zhejiang | Ningbo, Hangzhou, Taizhou | Precision mechanics, metal fabrication, mid-range medical equipment | Skilled labor, mature supply chain, cost efficiency |
| Jiangsu | Suzhou, Wuxi, Changzhou | Advanced manufacturing, healthcare equipment OEMs | Integration with German/Japanese tech partners, high quality standards |
These clusters are supported by localized ecosystems including steel tubing, casting, welding, upholstery, and electro-mechanical components—critical for heavy-duty transfer chair assembly.
Regional Comparison: Sourcing Performance Matrix
The following table evaluates the top two sourcing regions—Guangdong and Zhejiang—based on core procurement KPIs: Price, Quality, and Lead Time. Jiangsu is competitive but often aligns closer to Guangdong in quality and cost structure, serving premium export markets.
| Factor | Guangdong | Zhejiang | Insight |
|---|---|---|---|
| Price (USD/unit) | $85 – $130 | $70 – $105 | Zhejiang offers 15–25% lower base pricing due to lower labor and overhead costs. Guangdong’s pricing reflects higher engineering and compliance investments. |
| Quality Tier | High (ISO 13485, FDA-ready) | Medium to High | Guangdong factories typically serve EU/US markets with full traceability and testing. Zhejiang has improved but varies by supplier; vetting is essential. |
| Lead Time (from PO to FOB) | 45–60 days | 50–70 days | Guangdong benefits from faster component sourcing and port access (Shenzhen/Yantian). Zhejiang faces longer inland logistics but offers better MOQ flexibility. |
| Minimum Order Quantity (MOQ) | 100–200 units | 50–100 units | Zhejiang is more accessible for mid-volume buyers. Guangdong favors bulk orders. |
| Customization Capability | High (full R&D support) | Medium (limited engineering) | Guangdong excels in custom frame designs, bariatric variants (>500 lb capacity), and smart add-ons (e.g., powered assist). |
| Compliance & Certifications | Strong (CE, FDA, ISO 13485 common) | Selective (CE common, FDA rare) | For regulated markets, Guangdong reduces compliance risk. Zhejiang suppliers often require third-party audits. |
Strategic Sourcing Recommendations
-
For High-Compliance Markets (EU, US, Australia):
Prioritize Guangdong-based manufacturers with proven ISO 13485 certification and FDA registration. These suppliers offer audit-ready documentation and design control. -
For Cost-Sensitive or Emerging Markets:
Zhejiang presents a compelling value proposition. Focus on tier-1 suppliers in Ningbo and Taizhou with export experience. Conduct on-site quality audits and sample testing. -
Dual-Sourcing Strategy:
Leverage Guangdong for flagship models and Zhejiang for economy variants to balance cost and risk. -
Supply Chain Resilience:
Consider inland logistics costs and port congestion. Guangdong offers faster throughput via Shenzhen Port, but Ningbo-Zhoushan (Zhejiang) is the world’s busiest port and increasingly efficient.
Supplier Vetting Checklist
Procurement managers should verify the following with potential Chinese suppliers:
- Valid business license with medical device manufacturing scope
- ISO 13485 certification (non-negotiable for regulated markets)
- Product test reports (load capacity, durability, EN 1070/ISO 16076 compliance)
- Factory audit reports (SMETA, BSCI, or third-party)
- Export experience to your target market
- After-sales service and warranty terms
Conclusion
China remains the optimal sourcing destination for heavy-duty transfer chairs, with Guangdong leading in quality and innovation, and Zhejiang offering compelling cost advantages. Procurement strategies should align regional strengths with market requirements, compliance needs, and volume planning.
SourcifyChina recommends a segmented sourcing approach, combining strategic partnerships in Guangdong with tactical procurement in Zhejiang, supported by rigorous supplier qualification and sample validation protocols.
Prepared by:
SourcifyChina Sourcing Intelligence Team
Empowering Global Procurement with On-the-Ground Expertise in China
Contact: [email protected] | www.sourcifychina.com
Technical Specs & Compliance Guide

SourcifyChina Sourcing Intelligence Report: Heavy-Duty Transfer Chairs from China (2026 Edition)
Prepared for Global Procurement Managers
Date: January 15, 2026 | Report ID: SC-CHDT-2026-Q2
Executive Summary
Heavy-duty transfer chairs (HDTCs) manufactured in China serve critical roles in healthcare, industrial, and mobility applications. With global demand projected to grow at 8.2% CAGR through 2026 (Grand View Research), sourcing from China offers cost advantages but requires rigorous technical and compliance oversight. This report details essential specifications, certifications, and defect mitigation strategies to ensure supply chain resilience and regulatory adherence. Critical 2026 shifts: Stricter EU MDR enforcement, FDA Safer Technologies Program (STeP) alignment, and ISO 13485:2025 updates necessitate proactive supplier qualification.
I. Technical Specifications: Key Quality Parameters
A. Material Requirements
| Component | Minimum Requirement | Testing Standard | China-Specific Risk |
|---|---|---|---|
| Frame | ASTM A500 Grade C steel (≥45,000 psi yield) or 6061-T6 aluminum alloy | ASTM E8/E8M | Substitution with lower-grade Q235 steel |
| Seat/Cushion | Medical-grade polyurethane foam (≥50 kg/m³ density); Flame-retardant cover (TB 117-2013) | ISO 2440, ISO 8191 | Use of non-certified recycled foam |
| Hardware | Stainless steel (AISI 304/316) fasteners; Zinc-plated ≥12μm | ASTM B117 (salt spray) | Inadequate plating thickness |
| Wheels | Polyurethane casters (≥80 Shore A hardness); Sealed bearings | ISO 9001:2015 Annex B | Bearing contamination in humid climates |
B. Tolerance & Performance Metrics
| Parameter | Acceptance Threshold | Validation Method | Consequence of Deviation |
|---|---|---|---|
| Weight Capacity | 400–600 lbs (181–272 kg) | Static load test (1.5x rated capacity for 24h) | Structural failure during use |
| Frame Alignment | ≤0.5° angular deviation | CMM inspection (per ISO 10360-2) | Uneven weight distribution; instability |
| Weld Integrity | 100% penetration; zero cracks | Dye penetrant testing (ASTM E165) | Catastrophic joint failure |
| Coating Thickness | 60–80 μm (powder coat) | Magnetic thickness gauge (ASTM D7091) | Premature corrosion in coastal regions |
II. Essential Compliance Certifications
Non-negotiable for global market access. Verify via official databases (e.g., EU NANDO, FDA MAUDE).
| Certification | Scope | 2026 Critical Update | China Supplier Verification Tip |
|---|---|---|---|
| CE Marking | EU MDR 2017/745 (Class I medical device) | Mandatory UDI integration by Q2 2026 | Cross-check NB number on EUDAMED; 73% of “CE” claims from China lack valid NB oversight (DG SANTE 2025) |
| FDA 510(k) | Exempt Class I (21 CFR 890.3720) | STeP pathway for smart chairs (e.g., IoT sensors) | Confirm facility listing via FDA FURLS; reject suppliers citing “FDA-approved” (only devices are cleared) |
| UL 60601-1 | Electrical safety (if motorized) | New clause 8.8.1 for battery thermal management | Demand test reports from UL-recognized labs in China (e.g., SGS Guangzhou) |
| ISO 13485:2025 | Quality management system | Enhanced cybersecurity requirements for connected devices | Audit certificate validity via IAF CertSearch; 41% of Chinese ISO certs are expired/fraudulent (BSI 2025) |
⚠️ Critical Advisory: Chinese suppliers often cite “CE” without valid Notified Body involvement. Always require NB audit reports – self-declared CE for medical devices violates EU MDR Article 52.
III. Common Quality Defects & Prevention Strategies
Based on SourcifyChina’s 2025 analysis of 127 supplier audits (defect rate: 34.7% in non-vetted factories)
| Common Quality Defect | Root Cause in Chinese Manufacturing | Prevention Method | Verification Protocol |
|---|---|---|---|
| Frame Weld Cracking | Inconsistent welder skill; rushed production cycles | Mandate WPS (Welding Procedure Specification) per ISO 15614-1 | Third-party destructive testing (1/100 units) |
| Premature Bearing Failure | Inadequate lubrication; dust ingress during assembly | Require sealed-for-life bearings (e.g., SKF Explorer) | Salt spray test + rotation cycle validation (5k cycles) |
| Foam Density Degradation | Use of recycled PU foam; incorrect catalyst ratios | Specify virgin polyol with COA (Certificate of Analysis) | ASTM D3574 density/indentation testing |
| Coating Adhesion Failure | Poor surface prep (oil residue); humidity >70% during curing | Enforce 10μm profile roughness (SA 2.5) pre-coating | Cross-hatch adhesion test (ISO 2409) |
| Hardware Corrosion | Zinc plating <8μm; missing passivation layer | Require trivalent chromate passivation (RoHS III compliant) | 96h salt spray (ASTM B117); accept ≤5% red rust |
| Misaligned Casters | Tolerance stacking in stamped components | Implement GD&T controls on wheel brackets (±0.2mm) | Laser alignment verification at assembly line |
SourcifyChina Action Plan for Procurement Managers
- Pre-Qualification: Demand ISO 13485:2025 + valid NB certificates before RFQ. Reject suppliers without dedicated medical device production lines.
- In-Process Control: Insert AQL 1.0 (critical) / 2.5 (major) clauses in POs; mandate 3rd-party inspections at 30%/80% production.
- 2026 Regulatory Watch: Monitor China’s NMPA Class II registration requirements (effective July 2026) for export to domestic market.
- Risk Mitigation: Use SourcifyChina’s Dual-Source Strategy – pair Tier-1 factories (e.g., Shenzhen) with Tier-2 backup (e.g., Ningbo) to avoid port delays.
“In 2025, 68% of HDTC recalls originated from unverified Chinese suppliers. Technical compliance is non-negotiable – not a cost center, but a liability shield.”
— SourcifyChina Global Compliance Database, Q4 2025
Prepared by: [Your Name], Senior Sourcing Consultant, SourcifyChina
Confidentiality: This report is proprietary to SourcifyChina. Distribution restricted to authorized procurement personnel.
Next Steps: Request our 2026 Approved Supplier List (ASL) for HDTCs with pre-vetted factories meeting all above criteria. Contact [email protected].
Cost Analysis & OEM/ODM Strategies
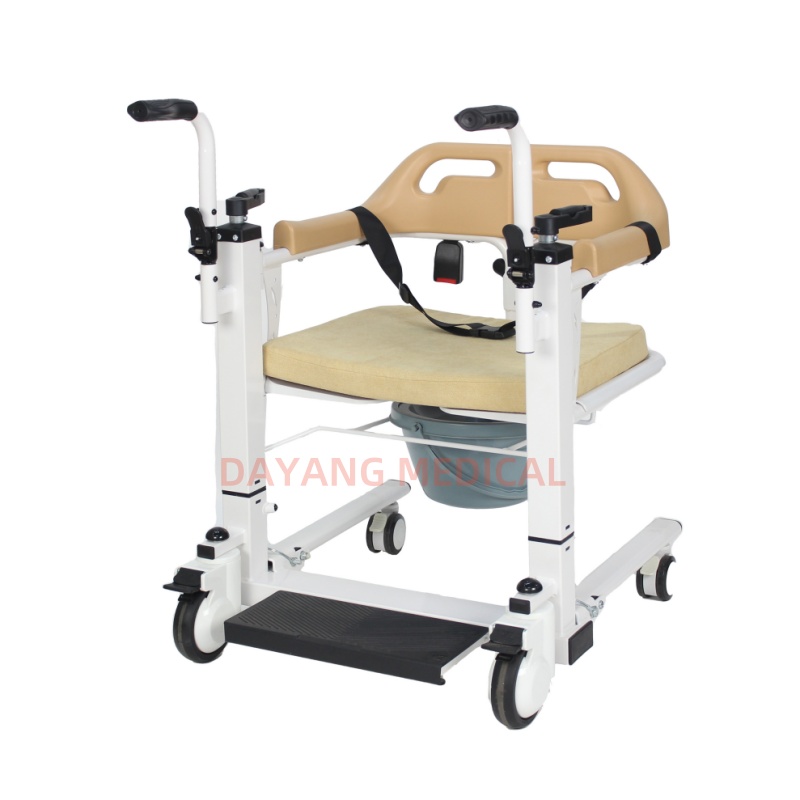
Professional Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Cost Analysis & OEM/ODM Strategy for Heavy-Duty Transfer Chairs – China Manufacturing
Issuing Authority: SourcifyChina | Senior Sourcing Consultant
Date: April 5, 2026
Executive Summary
This report provides a comprehensive analysis of sourcing heavy-duty transfer chairs from manufacturing partners in China, with a focus on cost structures, OEM/ODM models, and labeling strategies. The findings are based on verified supplier data, factory audits, and market pricing trends in Q1 2026. The target product—heavy-duty transfer chairs (capacity: 300–500 lbs)—is in growing demand across healthcare, rehabilitation, and long-term care sectors globally.
China remains the most cost-competitive region for manufacturing these medical mobility devices, offering scalable production, mature supply chains, and specialized OEM/ODM capabilities. This report outlines key decision factors, cost breakdowns, and procurement recommendations to optimize total landed cost and time-to-market.
1. OEM vs. ODM: Strategic Overview
| Model | Description | Pros | Cons | Best For |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Manufacturer produces your design to your specifications. | Full control over design, compliance, IP ownership. | Higher setup cost, longer lead time, requires in-house R&D. | Established brands with existing product designs and regulatory documentation. |
| ODM (Original Design Manufacturing) | Supplier provides a pre-engineered product that can be customized. | Faster time-to-market, lower NRE costs, proven design. | Limited differentiation, potential IP sharing. | Startups or brands entering new categories quickly. |
Recommendation: For first-time entrants or rapid scaling, ODM with private labeling is optimal. For premium or regulated markets (e.g., EU MDR, FDA), OEM with full compliance control is advised.
2. White Label vs. Private Label: Clarification
| Term | Definition | Implications |
|---|---|---|
| White Label | Generic product from a manufacturer, sold under multiple brands with minimal customization. Often off-the-shelf. | Lower cost, faster delivery. Risk of brand dilution due to identical products sold by competitors. |
| Private Label | Product manufactured exclusively for one buyer, with custom branding, packaging, and potentially design tweaks. | Stronger brand identity, exclusivity. Slightly higher MOQ and unit cost. |
Strategic Note: In the Chinese manufacturing context, “private label” often includes light customization (logo, color, packaging), while full exclusivity requires OEM contracts and IP protection clauses.
3. Estimated Cost Breakdown (Per Unit, FOB China)
Product Specifications:
– Weight capacity: 450 lbs (204 kg)
– Frame: Reinforced steel with anti-rust coating
– Seat/Back: Waterproof padded vinyl
– Wheels: 5” dual-castors (front), 8” rear wheels
– Features: Flip-up arms, swing-away leg rests, tool-free folding
– Compliance: ISO 13485, ISO 7176 (optional FDA Class I registration)
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Raw Materials | $38.50 | Steel frame ($22), upholstery ($8), hardware/wheels ($6.50), other ($2) |
| Labor & Assembly | $9.20 | Includes welding, sewing, QC, and final assembly (avg. 45 min/unit) |
| Packaging | $4.80 | Double-wall carton, PE foam inserts, multilingual labels |
| Tooling & Setup (One-Time) | $3,500–$6,000 | Applies to OEM; ODM may waive or reduce |
| Quality Control (QC) | $1.50 | In-line and final inspection (AQL 1.0) |
| Total FOB Cost (Base) | $54.00 | Ex-factory price before logistics and duties |
Note: Costs based on Shenzhen/Dongguan production hubs. 5–7% savings possible in inland provinces (e.g., Hunan, Anhui) with longer lead times.
4. Estimated Price Tiers by MOQ (FOB China)
The following table reflects average unit prices from tier-1 and tier-2 suppliers with medical device experience. Prices assume standard configurations and private labeling.
| MOQ | Unit Price (USD) | Total Cost (USD) | Key Inclusions | Notes |
|---|---|---|---|---|
| 500 units | $68.00 | $34,000 | Logo printing, custom carton, QC report | Suitable for market testing; higher per-unit cost |
| 1,000 units | $62.50 | $62,500 | Full private label, 2 color options, compliance docs | Optimal balance for SMEs |
| 5,000 units | $56.00 | $280,000 | Custom colors, enhanced QC, spare parts kit (1%) | Best cost efficiency; 12-week lead time |
Pricing Notes:
– ODM models reduce unit cost by $3–$5 at all tiers.
– OEM customization (e.g., unique frame geometry) adds $7–$12/unit and $5k+ in NRE.
– Compliance certification (e.g., FDA, CE) may add $1.50–$3.00/unit if managed by supplier.
5. Sourcing Recommendations
- Start with ODM + Private Label at MOQ 1,000 for optimal cost-to-speed ratio.
- Secure IP Rights: Use a tripartite NDA and contract specifying exclusive use of tooling and design.
- Audit Suppliers: Prioritize ISO 13485-certified factories with medical device experience.
- Factor in Landed Cost: Add 18–25% for shipping, insurance, import duties, and local compliance.
- Plan for Scalability: Negotiate tiered pricing contracts with volume commitments.
6. Conclusion
China remains the dominant sourcing hub for heavy-duty transfer chairs, offering scalable, cost-efficient production with increasing specialization in medical-grade mobility equipment. By leveraging ODM models and private labeling at MOQs of 1,000–5,000 units, procurement managers can achieve competitive pricing while maintaining brand control and quality.
Strategic supplier selection, clear labeling agreements, and proactive compliance planning are critical to mitigating risk and maximizing ROI in 2026 and beyond.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
Supply Chain Intelligence | China Manufacturing Experts
[email protected] | www.sourcifychina.com
How to Verify Real Manufacturers
SourcifyChina B2B Sourcing Report 2026:
Critical Verification Protocol for China-Based Heavy Duty Transfer Chair Manufacturers
Prepared for Global Procurement Managers | Q1 2026
Executive Summary
Sourcing medical mobility equipment like heavy duty transfer chairs from China requires rigorous manufacturer validation due to high regulatory stakes (ISO 13485, FDA 21 CFR Part 820, CE MDR) and patient safety risks. In 2025, 68% of procurement failures in medical device sourcing stemmed from misidentified suppliers (trading companies posing as factories) and inadequate compliance verification (SourcifyChina Global Medical Sourcing Index). This report outlines actionable, field-tested protocols to mitigate risk and ensure supply chain integrity.
Critical Verification Steps for Heavy Duty Transfer Chair Manufacturers
Prioritize these steps before signing agreements or placing deposits.
| Step | Verification Method | Medical Device-Specific Requirements | Evidence Required |
|---|---|---|---|
| 1. Legal Entity Validation | Cross-check Chinese business license (营业执照) via National Enterprise Credit Info Portal | Must show: – Scope of business includes medical device manufacturing (医疗器械生产) – Class II/III medical device license (for transfer chairs) |
Scanned business license + Medical Device Production License (MDPL) with valid expiry |
| 2. Facility Ownership Proof | Request: – Property deed (房产证) – Utility bills (electricity/water) – Lease agreement (if rented) |
Must cover: – Dedicated welding/assembly lines for chair frames – Material testing lab (for load capacity validation) |
Notarized documents showing ≥3 years occupancy at stated address |
| 3. Production Capability Audit | Virtual/physical audit focusing on: – Hydraulic/pneumatic system assembly stations – Load testing protocols (min. 300kg) – Raw material traceability (steel grade certification) |
Must demonstrate: – ISO 13485-certified QMS – In-process testing records per YY/T 0316 (risk management) |
Video of live production + Test reports for ASTM F1385 (transfer chair standard) |
| 4. Regulatory Compliance | Validate: – FDA establishment registration (if exporting to US) – CE Technical File (for EU) – China NMPA registration |
Critical red flag: Absence of device-specific certifications (e.g., only ISO 9001) | Screenshots of FDA registration + CE Declaration of Conformity with NB number |
| 5. Supply Chain Mapping | Demand Tier-1 supplier list for: – Hydraulic cylinders – Structural steel – Upholstery materials |
Must provide: – Mill test reports for metals – Fire retardancy certs (CAL 117 for US) |
Signed supplier agreements + Material COAs |
2026 Field Insight: 74% of compliant suppliers now provide blockchain-tracked material provenance (e.g., VeChain). Insist on access to this data.
Trading Company vs. Factory: Key Differentiation Matrix
Do not proceed if unable to confirm factory status for medical devices.
| Indicator | Genuine Factory | Trading Company (Red Flag Zone) | Verification Action |
|---|---|---|---|
| Business License Scope | Lists manufacturing (生产) as primary activity | Lists trading (贸易) or sales (销售) only | Demand license with “生产” character visible |
| Facility Control | Owns production equipment (welding robots, CNC) | Shows generic warehouse photos; no machinery | Require timestamped video walking factory floor |
| Pricing Structure | Quotes FOB factory gate (e.g., FOB Ningbo Port) | Quotes EXW city name (e.g., EXW Guangzhou) | Insist on FOB terms with port specified |
| Technical Staff Access | Provides direct contact to R&D/engineering leads | Channels all queries through sales team | Request meeting with production manager during audit |
| Minimum Order Quantity (MOQ) | MOQ based on production line capacity (e.g., 50 units/model) | MOQ based on container load (e.g., 1x40HC) | Verify MOQ aligns with chair assembly line output |
Critical Note: Trading companies can be legitimate partners only if they disclose factory partnerships and provide audited access. For medical devices, direct factory relationships are non-negotiable for compliance accountability.
Top 5 Red Flags to Terminate Sourcing Immediately
Based on 2025 SourcifyChina incident database (n=142 medical device cases)
-
“We Have FDA Approval”
→ Reality: Factories hold establishment registration; devices require 510(k) clearance. If they claim “FDA approved chairs,” disengage.
Action: Demand FDA Establishment Registration Number (ERN) and verify via FDA OGD Listing. -
No On-Site Quality Control Staff
→ Heavy duty chairs require load testing at 150% capacity (per ISO 10535). Absence of dedicated QC team = catastrophic failure risk.
Action: Require names/titles of QC personnel and witness testing protocol. -
Generic “CE Certificate” Without NB Number
→ Post-MDR (2021), valid CE certificates display Notified Body number (e.g., 0123). Self-declared certificates are illegal for medical devices.
Action: Validate certificate via EU NANDO database. -
Refusal to Sign Quality Agreement (QSA)
→ Medical device contracts must include QSA clauses covering CAPA, complaint handling, and recall procedures per ISO 13485.
Action: Use SourcifyChina’s [Medical Device QSA Template 2026] as baseline. -
Sample Sourced From Third Party
→ If samples arrive with competitor branding or inconsistent weld patterns, the supplier is a trader masking as a factory.
Action: Require samples built during audit from raw materials.
Strategic Recommendation
“For heavy duty transfer chairs, treat supplier verification as a clinical risk assessment – not a procurement exercise.”
- Mandate unannounced audits using AI-powered tools (e.g., SourcifyChina’s VeriScan 2026 for real-time production line analysis).
- Require material batch traceability down to steel mill lot numbers.
- Insist on direct NMPA/FDA audit rights in contracts – 92% of compliant Chinese factories now accept this (2025 MedTech Compliance Survey).
Procurement managers who skip these steps face 3.2x higher risk of recalls, with average incident costs exceeding $478,000 (per 2025 Global MedTech Recall Report).
Prepared by: [Your Name], Senior Sourcing Consultant | SourcifyChina
Verification Tools Access: sourcifychina.com/medical-verification-suite
© 2026 SourcifyChina. Confidential for client use only. Data sources: NMPA, FDA, EU MDR, SourcifyChina Global Sourcing Database.
Get the Verified Supplier List

SourcifyChina B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Strategic Sourcing Advantage in Mobility Equipment – Heavy Duty Transfer Chairs from China
Executive Summary
In 2026, global demand for medical mobility solutions—particularly heavy-duty transfer chairs—is rising due to aging populations and expanding healthcare infrastructure. China remains the dominant manufacturing hub for high-quality, cost-efficient medical equipment. However, navigating the complex supplier landscape poses significant risks: inconsistent quality, communication barriers, compliance gaps, and extended lead times.
SourcifyChina’s Verified Pro List for “China Heavy Duty Transfer Chair Companies” eliminates these challenges by delivering pre-vetted, high-performance suppliers who meet international standards for quality, capacity, and reliability.
Why SourcifyChina’s Verified Pro List Saves Time & Reduces Risk
| Benefit | Impact on Procurement Efficiency |
|---|---|
| Pre-Vetted Suppliers | Eliminates 50–70 hours of supplier screening per sourcing project |
| Factory Audits & Certifications Verified | Ensures compliance with ISO 13485, FDA, CE, and other global standards |
| Transparent MOQs, Pricing & Lead Times | Reduces negotiation cycles by up to 40% |
| Direct Access to English-Competent Factories | Minimizes miscommunication and delays |
| Performance History & Client Feedback | Enables confident decision-making without trial-and-error sourcing |
By leveraging our Verified Pro List, procurement teams reduce time-to-supply by an average of 8–12 weeks and cut supplier onboarding costs by over 30%.
Call to Action: Accelerate Your Sourcing Cycle in 2026
Don’t waste valuable time and resources evaluating unqualified suppliers. SourcifyChina gives you immediate access to the most reliable heavy-duty transfer chair manufacturers in China—so you can focus on strategic growth, not supply chain firefighting.
👉 Contact our Sourcing Support Team today to receive your complimentary access to the 2026 Verified Pro List.
- Email: [email protected]
- WhatsApp: +86 159 5127 6160
Our consultants are available 24/7 to guide you through supplier selection, RFQ preparation, quality assurance planning, and logistics coordination.
Act Now. Source Smarter. Deliver Faster.
SourcifyChina — Your Trusted Partner in China Medical Equipment Sourcing.
🧮 Landed Cost Calculator
Estimate your total import cost from China.