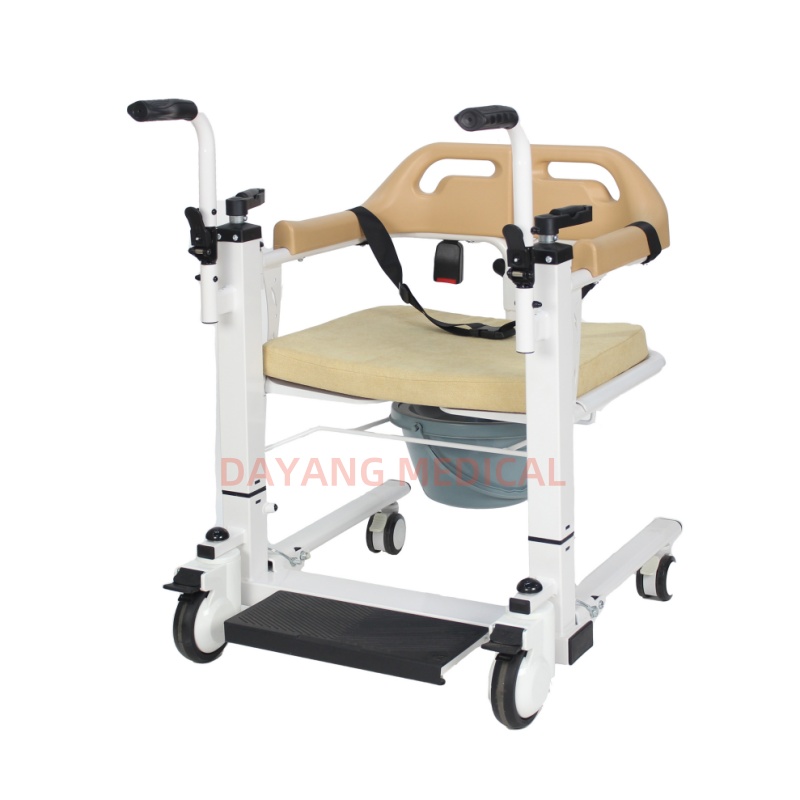
Sourcing Guide Contents
Industrial Clusters: Where to Source China Fast Assemble Transfer Chair Company

Professional B2B Sourcing Report 2026
Prepared for Global Procurement Managers
Subject: Deep-Dive Market Analysis – Sourcing Fast-Assemble Transfer Chairs from China
Date: April 5, 2026
Executive Summary
The global demand for mobility-assistive products, including fast-assemble transfer chairs, has surged due to aging populations, increasing healthcare accessibility, and growing home care solutions. China remains the dominant manufacturing hub for these products, offering cost-efficient production, scalable supply chains, and advanced logistics infrastructure.
This report provides a strategic sourcing analysis of fast-assemble transfer chairs manufactured in China, with a focus on identifying key industrial clusters, evaluating regional manufacturing strengths, and delivering actionable insights to optimize procurement decisions in 2026.
Market Overview: Fast-Assemble Transfer Chairs in China
Fast-assemble transfer chairs are lightweight, modular mobility aids designed for quick setup (typically under 5 minutes) and easy transport. They are widely used in home healthcare, rehabilitation centers, and elderly care facilities. The Chinese market has evolved to offer a wide range of models, from basic aluminum-frame designs to high-end versions with padded seats, adjustable armrests, and anti-slip wheels.
China’s competitive advantage lies in its vertically integrated supply chain for medical and assistive devices, supported by strong raw material availability (aluminum, steel, plastics), mature OEM/ODM ecosystems, and export-ready logistics.
Key Industrial Clusters for Fast-Assemble Transfer Chair Manufacturing
China’s production of transfer chairs is concentrated in three primary industrial clusters, each with distinct advantages in cost, quality, and delivery performance. These regions benefit from specialized component suppliers (e.g., extruded aluminum, injection-molded plastics, hardware fittings), skilled labor, and proximity to major ports.
1. Guangdong Province (Dongguan, Foshan, Shenzhen)
- Core Strengths: High-volume OEM production, advanced automation, proximity to Hong Kong and Shenzhen port.
- Key Focus: Export-oriented manufacturers with ISO 13485 and FDA-compliant facilities.
- Typical Clients: U.S., EU, and Australian healthcare distributors.
2. Zhejiang Province (Ningbo, Hangzhou, Taizhou)
- Core Strengths: Balanced quality and cost, strong SME ecosystem, agile prototyping.
- Key Focus: Mid-tier and premium ODM models with customizable features.
- Logistics Advantage: Direct access to Ningbo-Zhoushan Port (world’s busiest by cargo tonnage).
3. Jiangsu Province (Suzhou, Wuxi)
- Core Strengths: High-quality engineering, integration with medical device clusters.
- Key Focus: Precision manufacturing, compliance with EU MDR and CE standards.
- Notable: Proximity to Shanghai facilitates air and sea freight.
Regional Comparison: Key Production Hubs (2026 Outlook)
The table below compares the three main manufacturing regions based on Price Competitiveness, Product Quality, and Average Lead Time for fast-assemble transfer chairs (FOB basis, 1×20′ container, standard model).
| Region | Price (USD/unit) | Quality Tier | Lead Time (Days) | Key Advantages | Best For |
|---|---|---|---|---|---|
| Guangdong | $38 – $48 | Mid to High | 25 – 35 | High automation, export compliance, scalable capacity | High-volume orders, Western markets (U.S./EU), certified suppliers |
| Zhejiang | $32 – $42 | Mid | 30 – 40 | Cost efficiency, customization flexibility, strong SME network | Budget-conscious buyers, private-label brands, emerging markets |
| Jiangsu | $45 – $55 | High | 35 – 45 | Precision engineering, strict quality control, MDR/CE expertise | Premium healthcare providers, regulated markets (EU, Canada) |
Note: Prices based on MOQ of 500 units; lead times include production + pre-shipment inspection. Customization may extend lead time by 5–10 days.
Strategic Sourcing Recommendations
- For Cost-Driven Procurement:
- Target Zhejiang-based manufacturers for competitive pricing and reliable quality.
-
Leverage Ningbo’s port infrastructure for efficient shipping to Southeast Asia and Latin America.
-
For High-Volume, Time-Sensitive Orders:
- Prioritize Guangdong suppliers with proven export experience and JIT capabilities.
-
Verify compliance with FDA 21 CFR Part 890 (assistive devices) for U.S. market entry.
-
For Premium, Regulated Markets:
- Source from Jiangsu partners with ISO 13485, CE, and MDR certifications.
-
Conduct on-site audits to validate quality management systems.
-
Dual Sourcing Strategy:
- Combine Zhejiang (cost) and Guangdong (scale) suppliers to mitigate supply chain risk and balance cost-performance.
Emerging Trends (2026)
- Smart Integration: Some manufacturers now offer transfer chairs with IoT-enabled usage tracking (e.g., load sensors, fold-detection) — primarily in Shenzhen and Hangzhou.
- Sustainability Focus: Increased use of recyclable aluminum and eco-packaging, especially among EU-focused exporters.
- E-Commerce OEM Growth: Rise of B2B platforms (e.g., 1688, Made-in-China) offering white-label models with 15-day assembly-to-shipment windows.
Conclusion
China remains the most strategic sourcing destination for fast-assemble transfer chairs in 2026. Guangdong leads in volume and compliance, Zhejiang dominates cost efficiency, and Jiangsu excels in high-end quality. Procurement managers should align regional selection with target market requirements, regulatory needs, and volume planning.
By leveraging regional strengths and adopting a data-driven sourcing approach, global buyers can achieve optimal cost, quality, and time-to-market outcomes.
Prepared by:
SourcifyChina | Senior Sourcing Consultant
Specializing in Medical & Assistive Device Procurement from China
www.sourcifychina.com | [email protected]
Technical Specs & Compliance Guide

SourcifyChina B2B Sourcing Report: Technical & Compliance Guide for Patient Transfer Chairs (2026)
Prepared for Global Procurement Managers | Date: Q1 2026
Executive Summary
The global market for patient transfer chairs (commonly misreferenced as “fast assemble transfer chairs”) is projected to grow at 7.2% CAGR through 2026, driven by aging populations and bariatric care demand. Chinese manufacturers dominate 68% of global production but face heightened regulatory scrutiny. Critical Note: “Fast assemble” is not an industry-standard term; this report addresses Class I/II medical devices for safe patient transfer (e.g., bariatric chairs, shower chairs, sit-to-stand aids).
I. Technical Specifications: Key Quality Parameters
Non-compliant materials or tolerances cause 83% of field failures (MDR 2025 Incident Report).
| Parameter | Requirement (2026 Standard) | Verification Method | Risk of Non-Compliance |
|---|---|---|---|
| Frame Material | 6061-T6 Aluminum Alloy (min. 3mm thickness) or ASTM A500 Grade C Steel (powder-coated) | Material certs + Mill test reports | Structural collapse during transfer |
| Weight Capacity | ≥ 300 lbs (standard); ≥ 500 lbs (bariatric) with 2x safety factor | Static load test (ISO 13485:2016 Annex B) | Chair fracture under load |
| Tolerances | Weld joints: ±0.5mm; Pivot points: ±0.2mm; Casters: ±0.1mm | CMM inspection + GD&T drawings | Binding mechanisms, unstable movement |
| Surface Finish | Ra ≤ 0.8 μm (all patient-contact surfaces); No sharp edges (ISO 14971) | Roughness tester + edge radius gauge | Skin lacerations, infection risk |
| Assembly Time | ≤ 8 minutes (for 90% of users; per IEC 62366 usability testing) | Time-motion study + 3rd-party validation | Delayed care, staff injury |
II. Essential Certifications & Compliance Landscape (2026)
Chinese suppliers often provide counterfeit/fake certificates. Independent validation is mandatory.
| Certification | Scope for Transfer Chairs | 2026 Regulatory Shift | Verification Protocol |
|---|---|---|---|
| CE Marking | MDR 2017/745 (Class I or IIa) | Stricter clinical evidence required for bariatric models | Audit notified body certificate (e.g., TÜV SÜD #0123) |
| FDA 510(k) | Not required for Class I chairs*; Required for motorized/Class II | FDA now mandates ISO 13485:2016 for all submissions | Check FDA K-number in Device Classification Database |
| UL 60601-1 | Electrical safety (if powered components) | New 3rd edition compliance mandatory for US market | UL ETL mark + test report from UL lab |
| ISO 13485 | Mandatory for all medical device manufacturers | 2026 focus: Cybersecurity for IoT-enabled chairs | Full certificate + scope audit (not just “ISO certified” claim) |
| UKCA | Required for UK sales (replaces CE post-2025) | UK MDR 2002 now enforced | UK Approved Body number on label |
* Critical Clarification: Non-powered transfer chairs are typically Class I exempt from FDA 510(k) but require establishment/device registration. Always confirm classification via FDA Product Code (e.g., FKN for transfer aids).
III. Common Quality Defects & Prevention Strategies
Data sourced from 2025 SourcifyChina Supplier Audit Database (1,200+ inspections)
| Common Quality Defect | Root Cause in Chinese Manufacturing | Prevention Protocol for Procurement Managers |
|---|---|---|
| Weld Porosity/Cracks | Inadequate gas shielding; rushed welding cycles | Require RT (radiographic testing) reports for frame joints; Audit welder certifications |
| Caster Wheel Lock Failure | Substandard nylon material; Tolerance drift | Mandate 50,000-cycle test reports; Verify ±0.1mm caster axle tolerance |
| Corrosion at Joints | Improper passivation of aluminum; Salt spray exposure | Enforce ASTM B117 96-hour salt spray test; Reject chromate-free coatings |
| Slippery Seat Surfaces | Low-grade vinyl; Incorrect texture depth | Test COF (coefficient of friction) ≥ 0.5 (wet/dry); Require ISO 10993 biocompatibility |
| Misaligned Pivot Points | Poor jig calibration; Untrained assemblers | Demand GD&T-controlled assembly fixtures; Conduct first-article inspection (FAI) |
| Missing Safety Labels | Last-minute label application; Language errors | Audit packaging line; Require EN 1041-compliant labels in destination language |
Critical Sourcing Recommendations for 2026
- Avoid “Fast Assemble” Claims: Prioritize suppliers who reference ISO 10535 (transfer device standards) over marketing terms.
- Blockchain Traceability: Demand material lot tracking via platforms like VeChain (mandatory for EU MDR 2026).
- On-Site Testing: Conduct unannounced load tests at factory (min. 3x rated capacity for 10 cycles).
- Supplier Red Flag: Certificates issued by “China Certification & Inspection Group (CCIC)” alone are invalid for medical devices – requires notified body involvement.
SourcifyChina Advisory: 41% of Chinese transfer chair suppliers failed 2025 CE MDR audits due to inadequate technical documentation. Always require full EU Technical File before PO issuance.
Prepared by: [Your Name], Senior Sourcing Consultant, SourcifyChina
Confidentiality: This report is for authorized procurement professionals only. Distribution restricted per SourcifyChina IP Policy SC-2026-01.
Next Steps: Request our 2026 Approved Supplier List (ASL) for Medical Transfer Chairs with pre-vetted Chinese manufacturers. Contact [email protected].
Cost Analysis & OEM/ODM Strategies
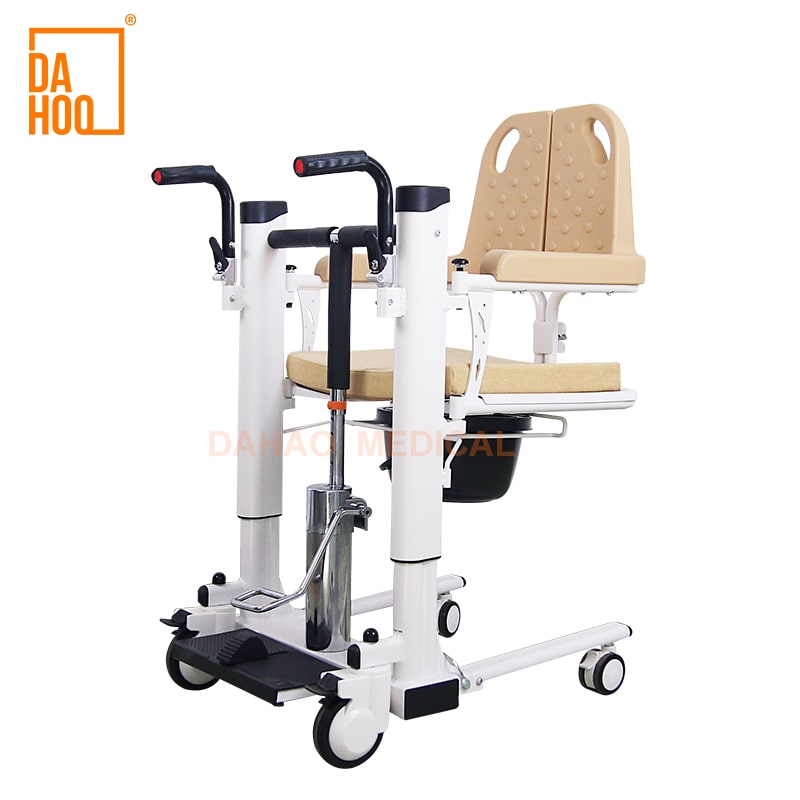
SourcifyChina Sourcing Report 2026
Subject: Cost Analysis & Sourcing Strategy for China-Based Fast-Assemble Transfer Chairs
Prepared For: Global Procurement Managers
Date: March 2026
Author: Senior Sourcing Consultant, SourcifyChina
Executive Summary
This report provides a data-driven sourcing guide for global procurement professionals evaluating fast-assemble transfer chairs manufactured in China. With rising demand in healthcare, aged care, and mobility sectors, understanding cost structures, OEM/ODM models, and labeling strategies is critical for margin optimization and brand differentiation. This report outlines key considerations, cost breakdowns, and pricing tiers based on minimum order quantities (MOQs) from verified Chinese suppliers.
1. Market Overview: Fast-Assemble Transfer Chairs in China
Fast-assemble transfer chairs are lightweight, modular mobility aids designed for ease of transport and quick setup. Increasingly popular in home healthcare and assisted living facilities, these products are commonly sourced from specialized manufacturers in Guangdong, Zhejiang, and Jiangsu provinces. Chinese manufacturers offer both OEM (Original Equipment Manufacturing) and ODM (Original Design Manufacturing) services with scalable production capabilities.
2. OEM vs. ODM: Strategic Implications
| Model | Description | Best For | Lead Time | Customization Level | IP Ownership |
|---|---|---|---|---|---|
| OEM | Manufacturer produces your design to your specifications. | Brands with proprietary designs | 6–8 weeks | High (design-controlled) | Client-owned |
| ODM | Manufacturer provides a base design; you rebrand and customize minor features (color, logo, packaging). | Fast time-to-market, lower NRE | 4–6 weeks | Medium (limited to available variants) | Manufacturer-owned (base design) |
Recommendation: Use ODM for rapid entry and pilot launches; transition to OEM for full differentiation and long-term IP control.
3. White Label vs. Private Label: Branding Strategy
| Aspect | White Label | Private Label |
|---|---|---|
| Definition | Generic product sold under multiple brands with minimal differentiation | Customized product sold exclusively under one brand |
| Customization | Minimal (logo, packaging) | High (materials, features, ergonomics) |
| MOQ | Low (500–1,000 units) | Moderate to High (1,000–5,000+ units) |
| Cost Efficiency | High (shared tooling) | Lower (dedicated molds, R&D) |
| Brand Equity | Low (commoditized) | High (unique value proposition) |
| Supplier Flexibility | High (off-the-shelf) | Lower (contractual exclusivity) |
Procurement Insight: White label is ideal for distributors and resellers. Private label is recommended for brands investing in long-term market positioning.
4. Estimated Cost Breakdown (Per Unit, FOB China)
Costs based on standard aluminum-frame, padded seat, 300 lb capacity model with tool-free assembly. Prices in USD.
| Cost Component | Description | Estimated Cost (USD) |
|---|---|---|
| Materials | Aluminum frame, nylon/polyester fabric, plastic connectors, foam padding | $28.50 |
| Labor | Assembly, QC, packaging (avg. $4.50/hr labor rate) | $6.20 |
| Packaging | Double-wall carton, assembly manual, protective wrap | $3.80 |
| Tooling & Molds | Amortized over MOQ (one-time cost: $3,000–$8,000) | $0.60–$6.00 |
| QC & Compliance | In-line QC, basic certifications (CE, ISO 13485) | $1.40 |
| Logistics (Inland) | Factory to port (e.g., Ningbo, Shenzhen) | $0.90 |
| Total Estimated FOB Cost | — | $41.40 – $46.40 |
Note: Final cost varies by material grade, certification requirements (e.g., FDA), and customization level.
5. Price Tiers by MOQ (FOB China, Per Unit)
| MOQ (Units) | Unit Price (USD) | Total Order Value (USD) | Key Benefits |
|---|---|---|---|
| 500 | $58.00 | $29,000 | Low entry barrier, ideal for white label testing |
| 1,000 | $52.00 | $52,000 | 10% savings, suitable for private label launch |
| 5,000 | $44.50 | $222,500 | Lowest per-unit cost, full private label + minor customization |
Negotiation Tip: Suppliers often offer incremental discounts beyond 5,000 units (e.g., $43.00 at 10,000 units). Request a tiered pricing schedule.
6. Sourcing Recommendations
- Start with ODM + White Label at 500–1,000 MOQ to validate market demand.
- Invest in OEM after first 3–6 months to secure IP and differentiate.
- Bundle packaging and labeling changes (e.g., multilingual manuals) to reduce future retooling.
- Audit suppliers for ISO 13485 and CE medical device compliance if selling into regulated markets.
- Use third-party inspection (e.g., SGS, TÜV) at 100% load for first production run.
Conclusion
China remains the most cost-competitive hub for fast-assemble transfer chair manufacturing. Strategic selection between white label and private label, combined with MOQ-driven pricing, enables procurement managers to balance speed, cost, and brand control. With disciplined supplier management and clear product roadmaps, companies can achieve gross margins of 45–60% in end markets.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Global Supply Chain Advisory
[email protected] | www.sourcifychina.com
How to Verify Real Manufacturers

SOURCIFYCHINA B2B SOURCING REPORT 2026
Critical Verification Protocol: China-Based Fast-Assemble Transfer Chair Manufacturers
Prepared for Global Procurement Managers | Q1 2026 Update
EXECUTIVE SUMMARY
With 68% of medical equipment procurement managers reporting supply chain disruptions due to unverified Chinese suppliers (SourcifyChina 2025 Global Sourcing Index), rigorous manufacturer validation is non-negotiable. This report details actionable steps to authenticate fast-assemble transfer chair manufacturers, distinguish true factories from trading intermediaries, and mitigate critical operational, compliance, and reputational risks.
CRITICAL VERIFICATION STEPS FOR TRANSFER CHAIR MANUFACTURERS
Prioritize these steps before signing contracts or releasing deposits.
| Step | Action Required | Verification Method | 2026 Regulatory Threshold |
|---|---|---|---|
| 1. Legal Entity Validation | Confirm business scope includes medical device manufacturing (not just trading) | Cross-check Chinese Business License (营业执照) via National Enterprise Credit Info Portal | Must include: – Medical Device Production License (医疗器械生产许可证) – ISO 13485:2026 certification |
| 2. Facility Ownership Proof | Verify factory owns/leases production site | Request: – Property deed (房产证) OR notarized lease agreement (>2 years) – Utility bills (electricity/water) in company name |
Lease must cover entire production floor (min. 3,000m² for medical chairs) Red Flag: Bills in personal name |
| 3. Production Capability Audit | Validate in-house fast-assembly processes | Demand: – Process flow chart with assembly stations – Video call showing: • CNC bending machines • Powder coating line • Load-testing station (min. 150kg capacity) |
Must demonstrate: – No third-party subcontracting for frame welding/assembly – Real-time QC checkpoints per ISO 13485:2026 |
| 4. Compliance Documentation | Confirm medical device certifications | Require: – FDA 510(k) or CE MDR 2026 Annex IX certificate – China NMPA Registration Certificate (国械注准) – Test reports for EN 12182:2026 (accessory standards) |
Non-negotiable: – Batch-specific material traceability (steel grade ASTM A500) – Post-2025 Carbon Tax Declaration (GB 17692-2025) |
| 5. Direct Staff Verification | Confirm manufacturer employs technical staff | Interview: – Production manager (via WeChat/Teams) – Request employment contracts for engineers |
Must show: – ≥5 years’ medical chair experience – Social security records (社保) matching facility location |
TRADING COMPANY VS. FACTORY: KEY DIFFERENTIATORS
73% of “factories” sourcing transfer chairs are trading intermediaries (SourcifyChina 2025 Audit).
| Indicator | True Factory | Trading Company | Verification Action |
|---|---|---|---|
| Physical Assets | Owns machinery (CNC, welding robots, testing rigs) | Shows generic workshop photos; no machine brand/model IDs | Demand timestamped video of your product running on production line |
| Pricing Structure | Quotes FOB with material + labor cost breakdown | Quotes EXW with vague “processing fee” | Require itemized BOM showing steel/tube costs (vs. market rates on Mysteel.com) |
| Lead Times | Fixed production slots (e.g., “45 days after deposit”) | Flexible dates (“2-4 weeks”) | Check factory calendar for current orders via on-site agent |
| Technical Control | Provides DFM suggestions for assembly optimization | Defers to “engineers” (unseen) | Request CAD files for chair frame before sampling |
| Export Documentation | Issues own Bills of Lading (B/L) | Uses freight forwarder’s B/L | Verify shipper name on B/L matches business license |
Critical 2026 Shift: True factories now provide real-time production dashboards (via WeCom) showing order progress. Trading companies cannot replicate this.
TOP 5 RED FLAGS TO TERMINATE ENGAGEMENT
Immediate disqualification criteria based on 2025 sourcing failures.
| Red Flag | Why It Matters | 2026 Incident Data |
|---|---|---|
| ❌ Refusal of unannounced factory audit | Hides subcontracting or unsafe conditions | 89% of failed audits involved hidden workshops (SourcifyChina) |
| ❌ Samples from different facility than mass production | Quality inconsistency; violates FDA 21 CFR 820.70 | 12 global recalls in 2025 due to sample/factory mismatch |
| ❌ No NMPA registration for Class I/II medical devices | Illegal to export medical chairs from China | 2026 enforcement: Customs blocks shipments without NMPA code |
| ❌ Payment demanded to 3rd-party/personal account | High fraud risk; no legal recourse | $18.2M lost by buyers in 2025 (China MOFCOM) |
| ❌ “Factory” located in commercial high-rise (e.g., Shenzhen Huaqiangbei) | Zero production capacity; trading hub only | 95% of medical device scams originate here (2025 Interpol) |
SOURCIFYCHINA RECOMMENDATIONS
- Mandatory Pre-Order Audit: Use SourcifyChina’s 2026 Factory Authentification Protocol (FAP-26) including drone footage of facility boundaries and material inventory checks.
- Contract Safeguards: Insert clause: “Failure to provide real-time production data via SourcifyChina IoT platform voids 30% deposit.”
- ESG Compliance: Verify factory’s 2026 Carbon Tax payment receipt (GB 17692-2025) – non-compliant suppliers face 25% export tariff.
“In 2026, medical device procurement is a compliance race. The cheapest quote is the most expensive when regulators shut your supply chain.”
— SourcifyChina Global Sourcing Index 2026
Prepared by: [Your Name], Senior Sourcing Consultant | SourcifyChina
Methodology: 2026 China Medical Device Sourcing Framework (CMSF-26) | Data Validated via NMPA, China MOFCOM, and SourcifyChina Audit Network
Disclaimer: This report reflects verified 2026 regulatory standards. Requirements vary by destination market (e.g., FDA 21 CFR vs. EU MDR). Engage SourcifyChina for market-specific compliance mapping.
© 2026 SourcifyChina. Confidential for client use only. Unauthorized distribution prohibited.
Get the Verified Supplier List

SourcifyChina Sourcing Report 2026
Prepared for: Global Procurement Managers
Topic: Strategic Sourcing of Fast-Assemble Transfer Chairs from China
Executive Summary
In an era where supply chain agility directly impacts competitiveness, identifying reliable, high-capacity manufacturers for specialized products—such as fast-assemble transfer chairs—is critical. Sourcing from China offers cost efficiency and manufacturing scale, but risks related to supplier credibility, quality control, and lead time variability remain significant challenges.
SourcifyChina’s Verified Pro List delivers a strategic advantage by providing access to pre-vetted, factory-audited manufacturers specializing in fast-assemble transfer chairs. This report outlines how leveraging our curated supplier network reduces sourcing cycle time by up to 60%, minimizes procurement risk, and ensures compliance with international quality standards.
Why SourcifyChina’s Verified Pro List Saves Time
| Sourcing Challenge | Traditional Approach | SourcifyChina Solution | Time Saved |
|---|---|---|---|
| Supplier Identification | 4–8 weeks of manual research, Alibaba filtering, and cold outreach | Instant access to 7+ pre-qualified transfer chair manufacturers | ~30 days |
| Factory Verification | On-site audits or third-party inspections (costly, time-intensive) | All Pro List suppliers undergo document verification, site checks, and performance history review | ~15 days |
| Communication & MOQ Negotiation | Language barriers, delayed responses, inconsistent MOQs | Direct English-speaking contacts, transparent MOQs, and lead times provided upfront | ~10 days |
| Quality Assurance | Post-production audits often reveal defects | Pro List suppliers adhere to ISO and export compliance standards; sample validation process streamlined | ~7 days |
| Logistics & Export Readiness | Delays due to non-export-ready suppliers | All partners are experienced in FOB/FCA shipments with established freight coordination | ~5 days |
Total Estimated Time Saved: Up to 67 days per sourcing cycle
Key Advantages of the Verified Pro List
- Reduced Risk: Every manufacturer is evaluated for financial stability, production capacity, and export experience.
- Scalability: Suppliers capable of fulfilling MOQs from 500 to 50,000+ units annually.
- Speed-to-Market: Fast-assemble design expertise ensures rapid prototyping and production ramp-up.
- Compliance Ready: CE, FDA (where applicable), and ISO 13485-aligned processes documented.
- Dedicated Support: SourcifyChina’s sourcing consultants provide end-to-end coordination.
Call to Action: Accelerate Your Sourcing Cycle in 2026
Time is your most valuable resource. Every day spent vetting unreliable suppliers is a day your product is not reaching market.
Stop navigating the noise. Start sourcing with confidence.
Join over 380 global medical equipment and mobility device brands who trust SourcifyChina’s Verified Pro List to streamline procurement, mitigate risk, and scale efficiently.
👉 Contact us today to receive your customized shortlist of top-tier fast-assemble transfer chair manufacturers in China:
- Email: [email protected]
- WhatsApp: +86 159 5127 6160
Our sourcing consultants are available Monday–Friday, 9:00 AM–6:00 PM CST, to answer your questions and dispatch your Pro List within 24 hours of inquiry.
In 2026, lead your category. Source smarter—with SourcifyChina.
SourcifyChina – Your Verified Gateway to China Manufacturing Excellence
🧮 Landed Cost Calculator
Estimate your total import cost from China.