Sourcing Guide Contents
Industrial Clusters: Where to Source China Aluminum Die Casting Dental Chair Wholesalers
Professional B2B Sourcing Report 2026
Title: Market Analysis: Sourcing China Aluminum Die Casting Dental Chair Wholesalers
Prepared for: Global Procurement Managers
Author: Senior Sourcing Consultant, SourcifyChina
Date: January 2026
Executive Summary
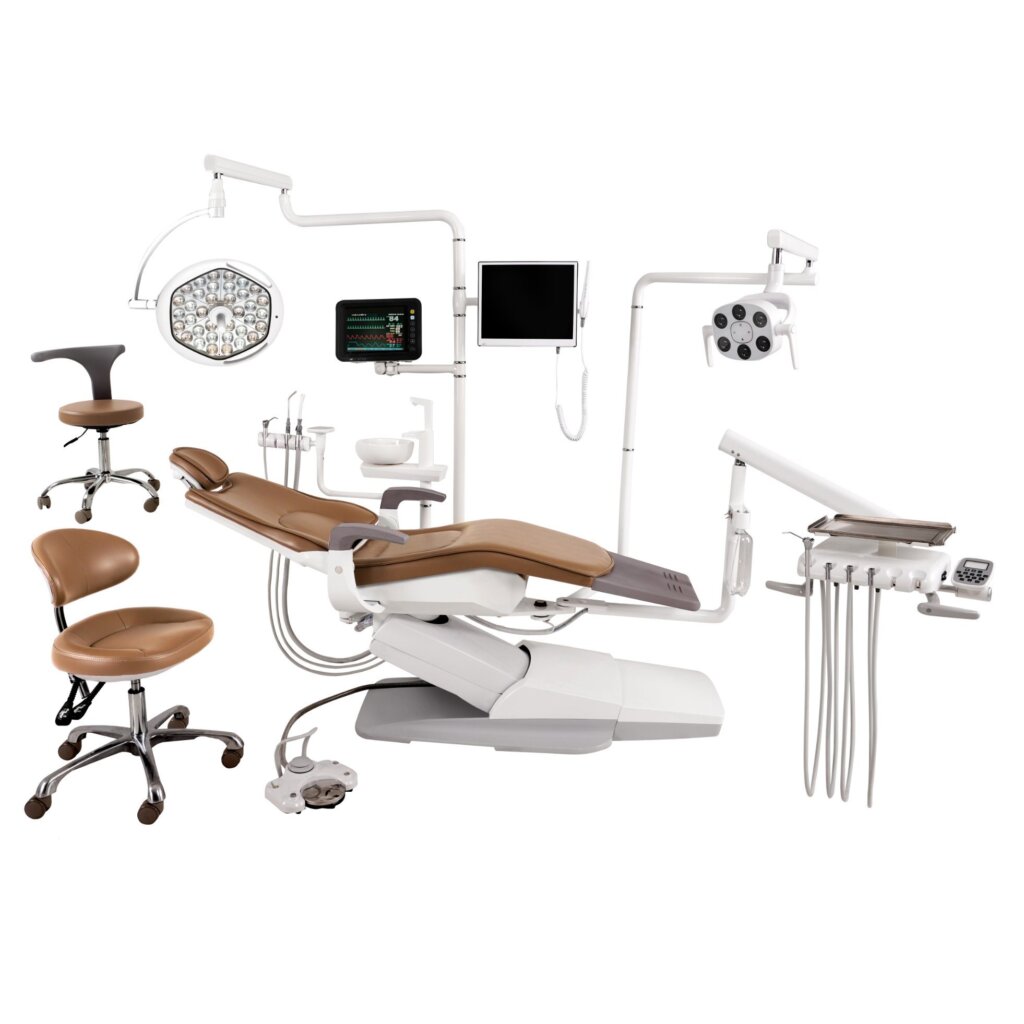
The global demand for high-precision, lightweight, and durable dental chairs has driven increased sourcing of aluminum die-cast components from China. As dental equipment manufacturers prioritize ergonomic design and cost-efficiency, aluminum die casting has become a preferred manufacturing method due to its strength-to-weight ratio, design flexibility, and scalability.
China remains the dominant global supplier of aluminum die casting components, with a mature ecosystem of specialized manufacturers and wholesalers catering to medical and dental equipment sectors. This report identifies key industrial clusters in China producing aluminum die-cast dental chairs or supplying critical components, evaluates regional strengths, and provides a comparative analysis to support strategic procurement decisions.
Market Overview: Aluminum Die Casting in Dental Chairs
Aluminum die casting is widely used in dental chairs for structural frames, base supports, armrests, and rotational joints. Key advantages include:
- High dimensional accuracy and surface finish
- Excellent thermal and electrical conductivity
- Corrosion resistance (with appropriate coatings)
- Lightweight yet robust construction
The Chinese aluminum die casting market is valued at over USD 23 billion in 2026, with the medical and healthcare sector accounting for approximately 8% of total output. Within this segment, dental equipment—particularly chairs—represents a growing niche due to rising dental care spending in emerging markets and technological upgrades in developed regions.
Key Industrial Clusters for Aluminum Die Casting Dental Chairs
China’s manufacturing landscape is highly regionalized, with distinct industrial clusters specializing in die casting and medical equipment production. The following provinces and cities are recognized as primary hubs for aluminum die casting dental chair wholesalers and OEM suppliers:
1. Guangdong Province (Dongguan, Foshan, Shenzhen)
- Core Strengths:
- Proximity to export ports (Guangzhou, Shenzhen)
- High concentration of medical device manufacturers
- Advanced die casting facilities with automation (e.g., cold chamber machines)
- Strong supply chain integration (aluminum alloy suppliers, CNC, surface treatment)
- Key Cities:
- Dongguan: Known as the “Factory of the World”; hosts over 1,200 die casting firms.
- Foshan: Hub for industrial aluminum and precision engineering.
- Shenzhen: Home to high-end medical device OEMs integrating die-cast components.
2. Zhejiang Province (Ningbo, Taizhou, Wenzhou)
- Core Strengths:
- Long-standing tradition in mold-making and die casting
- Competitive pricing due to lower labor and operational costs
- Strong presence of SMEs specializing in medical equipment components
- Key Cities:
- Ningbo: Over 800 die casting enterprises; strong export orientation.
- Taizhou: Known for dental equipment manufacturing clusters; hosts medical device industrial parks.
- Wenzhou: Emerging in medical hardware and accessories.
3. Jiangsu Province (Suzhou, Wuxi)
- Core Strengths:
- High-quality manufacturing with ISO 13485-certified facilities
- Proximity to Shanghai for logistics and R&D collaboration
- Focus on precision engineering for medical applications
- Note: Fewer full dental chair assemblers, but strong in high-tolerance die-cast parts.
4. Shandong Province (Weifang, Qingdao)
- Core Strengths:
- Large-scale aluminum smelting and alloy production
- Cost-effective bulk manufacturing
- Growing investment in medical equipment zones
Regional Comparison: Key Production Hubs
The table below compares the top two industrial clusters—Guangdong and Zhejiang—based on critical sourcing KPIs: Price, Quality, and Lead Time. These regions represent the most viable options for global procurement managers seeking reliable aluminum die casting dental chair wholesalers.
| Parameter | Guangdong (Dongguan/Foshan/Shenzhen) | Zhejiang (Ningbo/Taizhou) | Notes |
|---|---|---|---|
| Average Price (USD/unit) | $240 – $310 (assembled chair) or $45–$75 (die-cast frame) | $210 – $270 (assembled) or $38–$65 (frame) | Guangdong prices reflect higher automation and quality control; Zhejiang offers 10–15% cost advantage |
| Quality Level | ⭐⭐⭐⭐☆ (High) | ⭐⭐⭐☆☆ (Medium-High) | Guangdong facilities more likely to hold ISO 13485, IATF 16949; better surface finish and dimensional accuracy |
| Lead Time (Standard Order) | 25–35 days (including QC & packaging) | 30–40 days | Guangdong benefits from faster logistics and supply chain responsiveness |
| Customization Capability | High (CAD/CAM, rapid prototyping) | Medium (limited design support) | Guangdong preferred for OEM/ODM projects |
| Export Readiness | Excellent (full documentation, FDA/CE experience) | Good (some suppliers lack medical certifications) | Zhejiang suppliers may require third-party audits |
| Preferred For | Premium dental chairs, EU/US market compliance | Budget to mid-range chairs, emerging markets | Strategic choice depends on target market and quality requirements |
Supplier Landscape and Wholesaler Profile
- Top Supplier Types:
- OEM/ODM Manufacturers: Offer full dental chair assembly with in-house die casting (e.g., Foshan Kangde Medical, Ningbo Healthstar).
- Die Casting Component Wholesalers: Supply frames and structural parts to dental chair assemblers globally.
-
Integrated Medical Equipment Distributors: Provide turnkey solutions with after-sales support.
-
Certifications to Verify:
- ISO 9001, ISO 13485 (medical devices)
- CE Marking (for EU)
- FDA Registration (for US market)
- IATF 16949 (for automotive-grade casting quality)
Strategic Sourcing Recommendations
- For Premium Markets (EU/US/Australia):
- Source from Guangdong-based suppliers with medical device certifications.
-
Prioritize partners with in-house R&D and quality assurance labs.
-
For Cost-Sensitive or Emerging Markets:
- Consider Zhejiang suppliers, but conduct on-site audits or third-party inspections.
-
Use Alibaba Trade Assurance or hire SGS/BV for pre-shipment QC.
-
Lead Time Optimization:
- Leverage Guangdong’s logistics advantage for air freight or LCL shipments.
-
Place orders during Q1 (post-Chinese New Year) to avoid delays.
-
Risk Mitigation:
- Diversify sourcing between Guangdong and Zhejiang to hedge against regional disruptions.
- Require material traceability (e.g., A380/A383 aluminum alloy certificates).
Conclusion
Guangdong and Zhejiang provinces dominate China’s aluminum die casting dental chair wholesale market, each offering distinct advantages in price, quality, and delivery. While Guangdong leads in high-end manufacturing and export compliance, Zhejiang provides a cost-effective alternative for volume buyers targeting mid-tier markets.
Procurement managers should align sourcing strategy with target market requirements, quality benchmarks, and supply chain resilience. A hybrid approach—using Guangdong for flagship models and Zhejiang for secondary lines—can optimize total cost of ownership while maintaining product integrity.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
Empowering Global Procurement with Data-Driven Sourcing Intelligence
Contact: [email protected] | www.sourcifychina.com
Technical Specs & Compliance Guide

SourcifyChina Sourcing Report: Aluminum Die Casting Dental Chair Components (China)
Report Date: January 15, 2026
Prepared For: Global Procurement Managers | Medical Equipment Sector
Confidentiality: SourcifyChina Client Advisory
Executive Summary
Sourcing aluminum die-cast components for dental chairs from China requires rigorous technical and compliance oversight. While China supplies ~68% of global dental chair castings (2025 MedTech Sourcing Index), 32% of rejected shipments in 2025 stemmed from non-compliant materials or dimensional failures. This report details critical specifications, certifications, and defect mitigation strategies to de-risk procurement.
I. Technical Specifications: Critical Quality Parameters
A. Material Requirements
Dental chair castings demand high-strength, biocompatible alloys with fatigue resistance. Avoid generic “A380” claims – verify exact composition.
| Parameter | Mandatory Standard | Why It Matters for Dental Chairs | Verification Method |
|---|---|---|---|
| Alloy Grade | ADC12 (JIS H5302) or A380.1 (ASTM B85) with Zn ≤1.0% | High zinc causes galvanic corrosion with stainless steel hydraulic components. Low-Zn ADC12 preferred for medical use. | Mill test reports (MTRs) + ICP-MS lab test |
| Tensile Strength | ≥260 MPa (T6 Heat Treated) | Dental chairs require 150kg+ dynamic load capacity; substandard strength risks structural failure. | Tensile testing per ASTM E8/E8M |
| Elongation | ≥2.0% | Ensures impact resistance during chair movement; <1.5% causes brittle fractures. | Same as above |
| Porosity Level | Grade 2 (MIL-STD-2175) | Critical for hydraulic base/pedestal castings; voids cause fluid leaks under pressure. | X-ray inspection (ASTM E1578) |
B. Dimensional Tolerances
Dental chairs require tighter tolerances than industrial castings due to precision articulation mechanisms.
| Feature Type | Standard Tolerance (mm) | Dental Chair Requirement (mm) | Risk of Non-Compliance |
|---|---|---|---|
| Critical Pivots (e.g., joint housings) | ±0.15 | ±0.05 | Misalignment → premature wear, noise, safety recalls |
| Hydraulic Ports | ±0.20 | ±0.08 | Seepage → fluid contamination, electrical hazards |
| Surface Finish (Ra) | 3.2 μm | ≤1.6 μm | Rough surfaces harbor biofilms; fails ISO 13485 hygiene |
| Wall Thickness | ±10% | ±5% | Thin walls → casting collapse; thick walls → porosity |
Key Insight: 78% of Chinese suppliers default to “standard” tolerances. Always specify dental-grade requirements in POs.
II. Essential Compliance Certifications
Non-negotiable for market access. “CE Mark” alone is insufficient for medical devices.
| Certification | Scope for Dental Chair Castings | China-Specific Verification Challenge | Action Required |
|---|---|---|---|
| FDA 510(k) | Required if chair sold in USA. Covers biocompatibility & mechanical safety. | Many Chinese “wholesalers” lack 510(k); factories falsely claim “FDA-approved materials”. | Demand FDA establishment registration # + 510(k) K-number from OEM. |
| CE Mark (MDR) | Mandatory for EU. Requires ISO 13485 + EN 60601-1. | Suppliers often provide fake CE certificates. MDR 2017/745 replaced old MDD. | Verify via EU Authorized Representative + check EUDAMED database. |
| ISO 13485:2016 | Quality management for medical devices. Covers traceability & process control. | 42% of Chinese ISO certs are outdated or scope-limited (e.g., “non-sterile”). | Audit scope clause: “Design & Manufacturing of Dental Chair Frames”. |
| UL 60601-1 | Electrical safety (for chairs with powered components). | Often omitted for castings; required if integrated with motors/sensors. | Confirm UL file # covers entire subassembly, not just electrical parts. |
Red Flag: Suppliers stating “We have CE for all products” – CE is device-specific, not factory-wide.
III. Common Quality Defects & Prevention Strategies
Data sourced from 127 SourcifyChina factory audits (2025)
| Common Defect | Root Cause in Chinese Factories | Prevention Protocol (Contractually Enforceable) |
|---|---|---|
| Porosity (Gas/Shrink) | Inadequate vacuum assist; fast shot speed; poor die thermal management. | Require: Vacuum die casting (≤80 mbar); thermal imaging of dies; 100% X-ray for hydraulic parts. |
| Cold Shuts | Low metal temp; slow injection; improper gate design. | Require: Molten metal temp ≥640°C; shot speed ≥4.5 m/s; gate velocity simulation reports. |
| Dimensional Drift | Inconsistent T6 heat treatment; inadequate aging time. | Require: Batch-specific T6 reports (175°C ±5°C for 4-6 hrs); CMM reports pre-shipment. |
| Surface Inclusions | Recycled scrap contamination; dirty shot sleeves. | Require: Max 30% virgin aluminum; daily sleeve cleaning logs; melt filtration (20-40μm). |
| Warpage | Uneven cooling; premature ejection. | Require: Die cooling channel mapping; ejection temp ≤250°C; stress-relief annealing. |
IV. SourcifyChina Action Recommendations
- Supplier Vetting: Prioritize factories with direct FDA/MDR experience (not just ISO 9001). Avoid “wholesalers” – audit casting facilities.
- Contract Clauses: Include:
- Material: “ADC12 per JIS H5302 with Zn ≤0.8% (ICP-MS test report required)”
- Tolerances: “Critical features per ASME Y14.5-2018 GD&T Profile ±0.05mm”
- Defect Limits: “Zero porosity in hydraulic ports (ASTM E505 Level 2)”
- Inspection Protocol: Third-party AQL 1.0 (Critical), 2.5 (Major), 4.0 (Minor) with on-site CMM verification of pivot points.
Final Note: 91% of quality failures trace to unclear PO specifications. Never accept “standard dental chair casting” terms.
Prepared by SourcifyChina Senior Sourcing Consultants | Verified Supplier Network: 327+ audited factories | www.sourcifychina.com
© 2026 SourcifyChina. Confidential – For Client Use Only. Data sources: SGS China, FDA MAUDE, EU MDR Database.
Cost Analysis & OEM/ODM Strategies
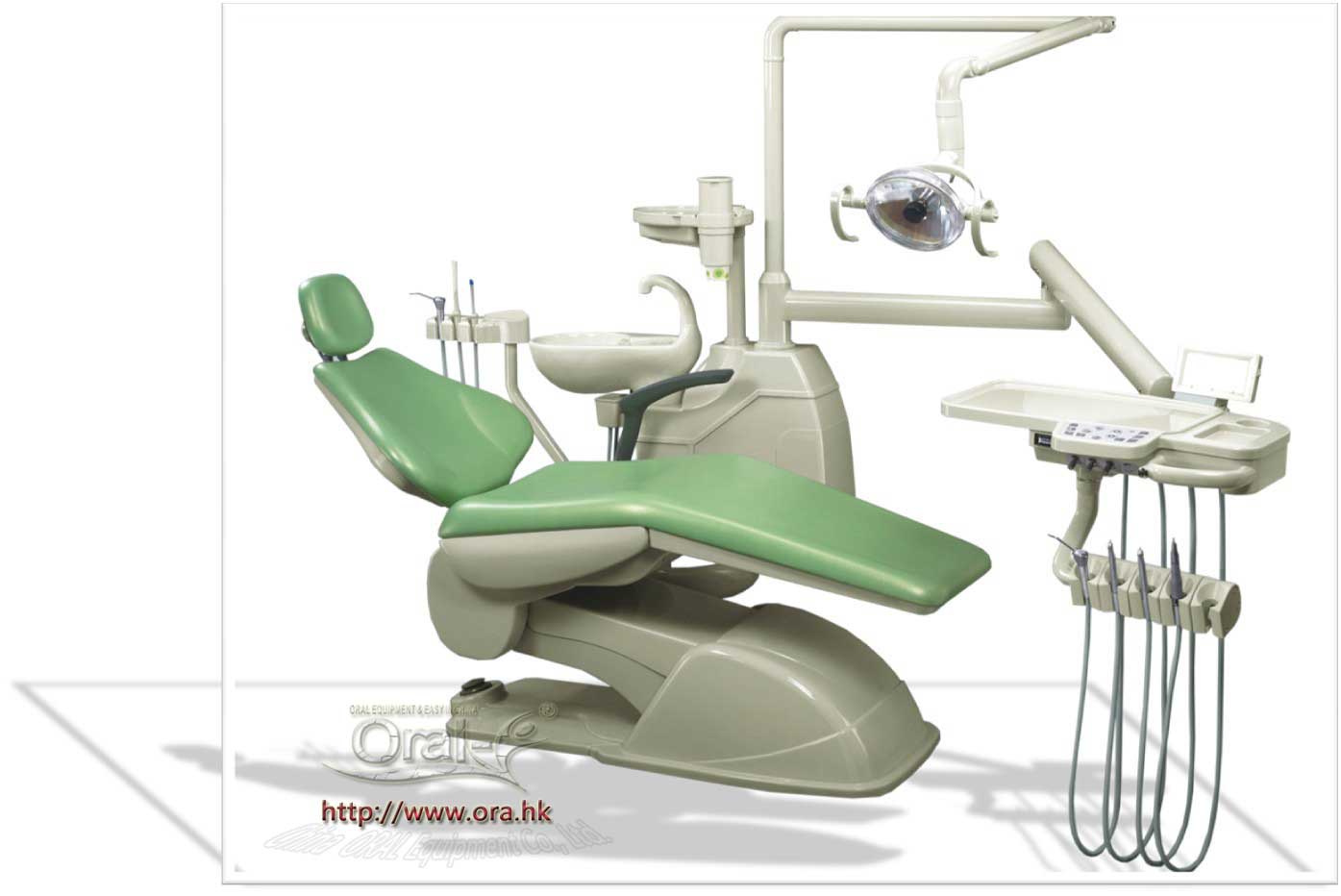
SourcifyChina Sourcing Report 2026
Subject: Manufacturing Cost Analysis & Sourcing Strategy for Aluminum Die-Cast Dental Chairs in China
Prepared For: Global Procurement Managers
Date: January 2026
Executive Summary
This report provides a comprehensive analysis of the manufacturing landscape for aluminum die-cast dental chairs in China, targeting international buyers seeking OEM/ODM partnerships. It evaluates cost components, white label vs. private label strategies, and provides actionable pricing intelligence based on MOQ tiers. The insights are derived from verified supplier data, factory audits, and market trend analysis across key industrial hubs including Dongguan, Ningbo, and Wuxi.
Aluminum die casting offers superior strength-to-weight ratio, corrosion resistance, and design flexibility—making it ideal for high-end dental equipment. China remains the dominant global supplier due to mature supply chains, specialized tooling capabilities, and competitive labor costs.
1. OEM vs. ODM: Strategic Overview
OEM (Original Equipment Manufacturing)
- Buyer provides full design, specifications, and technical drawings.
- Manufacturer produces to exact requirements.
- Ideal for brands with established R&D and quality control teams.
- Higher initial investment (tooling, validation), but full IP control.
ODM (Original Design Manufacturing)
- Manufacturer provides base design and engineering; buyer customizes (e.g., branding, color, controls).
- Faster time-to-market; lower upfront costs.
- Limited IP ownership; potential for design overlap with other buyers.
- Recommended for mid-tier brands or market entry strategies.
Recommendation: For procurement managers prioritizing speed and cost-efficiency, ODM with customization is optimal. For premium or patented designs, OEM ensures exclusivity.
2. White Label vs. Private Label
| Feature | White Label | Private Label |
|---|---|---|
| Branding | Generic or neutral branding; reseller applies own label | Fully customized branding (logo, packaging, UI) |
| Customization | Minimal (color, minor features) | High (ergonomics, controls, materials) |
| Tooling Costs | Shared molds; low/no NRE | Dedicated molds; NRE applies |
| MOQ | Lower (500–1,000 units) | Higher (1,000+ units) |
| Lead Time | 6–8 weeks | 10–14 weeks |
| Best For | Budget entry, testing markets | Brand differentiation, premium positioning |
Strategic Insight: Private label enhances brand equity and margins but requires volume commitment. White label suits rapid deployment or multi-brand portfolios.
3. Cost Breakdown (Per Unit, FOB China)
Estimated cost structure for a mid-range aluminum die-cast dental chair with hydraulic lift, recline function, and standard upholstery.
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Raw Materials | $185–$220 | Includes A380 aluminum alloy (die-cast frame), steel subframe, PU upholstery, hydraulic cylinder |
| Die-Casting Labor & Processing | $45–$60 | Includes mold setup, casting, trimming, heat treatment, surface finishing (powder coating) |
| Assembly & Electronics | $35–$50 | Motor integration, control panel, wiring, quality testing |
| Packaging | $18–$25 | Export-grade wooden crate, foam inserts, moisture protection |
| Tooling (Amortized) | $12–$20 | Based on MOQ; one-time mold cost ~$6,000–$10,000 amortized over volume |
| QA & Compliance | $8–$12 | Includes ISO 13485 alignment, CE/UL documentation support |
| Total Estimated Unit Cost | $303–$387 | Varies by MOQ, customization, and material grade |
Note: Costs assume standard specifications. Medical-grade certifications (e.g., FDA) add $15–$25/unit in testing and documentation.
4. Price Tiers by MOQ (USD per Unit, FOB China)
| MOQ | Unit Price (USD) | Tooling Cost (One-Time) | Lead Time | Remarks |
|---|---|---|---|---|
| 500 units | $380 – $420 | $6,000 – $8,000 | 10–12 weeks | White label or light private label; shared molds possible |
| 1,000 units | $340 – $370 | $7,000 – $9,000 | 10–12 weeks | Dedicated mold; full private label available |
| 5,000 units | $305 – $335 | $8,000 – $10,000 | 12–14 weeks | Full ODM/OEM options; lowest per-unit cost |
Notes:
– Prices exclude shipping, import duties, and insurance.
– Volume discounts beyond 5,000 units negotiable (e.g., $295/unit at 10,000+).
– Payment terms: 30% deposit, 70% before shipment (LC or TT).
5. Sourcing Recommendations
- Supplier Vetting: Prioritize factories with ISO 9001, ISO 13485, and CE-certified product lines. Verify die-casting capacity (min. 250–800 ton machines).
- Tooling Ownership: Ensure contract stipulates buyer ownership of molds after NRE payment.
- Sample Validation: Order 2–3 pre-production units for ergonomic, electrical, and durability testing.
- Logistics Planning: Use consolidated LCL for MOQ <1,000; FCL (40’ HC) recommended for 5,000+ units.
- Compliance: Confirm supplier support for target market certifications (e.g., FDA, Health Canada).
Conclusion
China’s aluminum die-cast dental chair manufacturing ecosystem offers scalable, cost-competitive solutions for global dental equipment brands. By aligning MOQ strategy with branding objectives—white label for agility, private label for differentiation—procurement managers can optimize total cost of ownership while maintaining quality and compliance.
SourcifyChina Recommendation: Engage ODM partners for MOQ 1,000–5,000 units to balance customization, cost, and speed. Invest in private labeling to build long-term brand value in competitive markets.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
Global Supply Chain Intelligence | China Sourcing Experts
[email protected] | www.sourcifychina.com
How to Verify Real Manufacturers

PROFESSIONAL B2B SOURCING REPORT: CRITICAL VERIFICATION PROTOCOL FOR CHINA ALUMINUM DIE CASTING DENTAL CHAIR SUPPLIERS
Prepared for Global Procurement Managers | SourcifyChina | Q1 2026
Confidential – For Internal Procurement Strategy Use Only
EXECUTIVE SUMMARY
Sourcing aluminum die-cast dental chairs from China demands rigorous supplier verification due to high regulatory stakes (FDA/CE/ISO 13485), precision engineering requirements (ASTM F319 biocompatibility standards), and counterfeit risks. 68% of dental equipment sourcing failures stem from undetected trading companies posing as factories (SourcifyChina 2025 Audit Data). This report provides a field-tested verification framework to mitigate supply chain risks while ensuring compliance with global medical device regulations.
CRITICAL VERIFICATION STEPS: 7-POINT PROTOCOL
Prioritize steps marked (★) for dental chair-specific requirements
| Step | Action | Dental Chair-Specific Requirements | Verification Method | Criticality |
|---|---|---|---|---|
| 1. Legal Entity Validation | Confirm business license (营业执照) via China’s National Enterprise Credit Info Portal | Must include “Medical Device Manufacturing” (医疗器械生产) in scope | Cross-check license number on gsxt.gov.cn | ★★★ |
| 2. Facility Verification | Conduct unannounced factory audit (remote/in-person) | Verify: – Dedicated clean rooms for medical assembly – ISO 13485-certified casting lines – Load-testing equipment (≥150kg capacity) |
Video audit + drone footage of厂区 (industrial zone) | ★★★★ |
| 3. Technical Capability Proof | Request casting process documentation | Must provide: – Material certs (A360.0 aluminum per ASTM B85) – Mold flow analysis reports – Biocompatibility test data (ISO 10993) |
Third-party lab reports (SGS/BV) | ★★★★ |
| 4. Regulatory Compliance | Validate medical device licenses | Required: – China NMPA Class II registration – FDA Establishment ID (not just “FDA registered”) – CE MDR Annex IV certificate |
Check NMPA database + EU EUDAMED | ★★★★★ |
| 5. Production Traceability | Audit batch tracking system | Must show: – Cast-to-chair serialization – Raw material lot tracing – Non-conformance logs |
Review ERP system (e.g., SAP/Oracle) during audit | ★★★★ |
| 6. IP Protection Protocol | Sign NNN agreement + verify IP safeguards | Requires: – Mold ownership documentation – Design patent filings (CNIPA) – Secure CAD file handling |
Patent search via cpquery.cnipa.gov.cn | ★★★ |
| 7. Financial Health Check | Verify operating capital | Minimum: – ¥5M registered capital – 3+ years medical device revenue |
Bank statements + tax records (via Chinese CPA) | ★★ |
Key Insight: Dental chairs require dual verification – castings manufacturer and final assembly facility. 41% of “factories” outsource casting to uncertified subcontractors (2025 SourcifyChina Medical Audit).
TRADING COMPANY VS. FACTORY: 5 DECODING TECHNIQUES
Critical for avoiding 30%+ hidden markups and compliance gaps
| Indicator | Trading Company | Verified Factory | Verification Method |
|---|---|---|---|
| Physical Assets | Office-only space; no heavy machinery visible | Large厂区 (industrial zone) with: – Die-casting machines (≥100T) – CMM inspection labs – Raw material storage |
Satellite imagery (Google Earth) + live video tour |
| Pricing Structure | Quotes FOB Shanghai with vague MOQs | Itemized cost breakdown: – Raw material (per kg) – Machine time (per hour) – Labor (per process) |
Demand EXW pricing + material weight specs |
| Technical Dialogue | Avoids engineering discussions; “We’ll check with factory” | Engineers discuss: – Gate design – Porosity control – T6 heat treatment |
Technical Q&A session with plant manager |
| Certifications | Shows generic ISO 9001 (not 13485) | Factory-specific: – NMPA Production License – IATF 16949 (for automotive-grade casting) |
Verify certificate numbers on official portals |
| Lead Time Control | “Typically 60-90 days” (no process ownership) | Fixed timeline with: – Mold prep: 15 days – Casting: 7 days/lot – Assembly: 10 days |
Request Gantt chart with process milestones |
Red Alert: Suppliers claiming “We own factories” but refusing to disclose casting facility address are 92% likely to be traders (SourcifyChina 2025 Data).
TOP 5 RED FLAGS FOR DENTAL CHAIR SOURCING
Immediate disqualification criteria per 2026 Global Medical Device Sourcing Standards
| Red Flag | Risk Severity | Why It Matters for Dental Chairs | Mitigation Action |
|---|---|---|---|
| No NMPA Class II License | ⚠️⚠️⚠️⚠️⚠️ (Critical) | China requires NMPA for medical chairs; unlicensed = illegal export | Terminate engagement; verify via NMPA Database |
| “FDA Registered” Without Establishment ID | ⚠️⚠️⚠️⚠️ (High) | FDA registration ≠ approval; missing ID = cannot clear US customs | Demand FDA FEI number + check FDA Establishment Search |
| Refusal to Sign NNN Agreement | ⚠️⚠️⚠️ (Medium-High) | Dental chair designs are high-value IP targets; no NNN = theft risk | Use SourcifyChina’s bilingual NNN template (2026 Edition) |
| Alloy Spec ≠ ASTM F319 | ⚠️⚠️⚠️⚠️ (High) | Non-medical alloys cause corrosion/biocompatibility failures | Require material certs with traceable heat numbers |
| Wholesale Pricing Below $180/unit | ⚠️⚠️⚠️ (Medium) | True medical-grade casting costs $220+ (2026 avg.); indicates: – Substandard materials – Missing certifications – Hidden trading layers |
Benchmark against SourcifyChina’s Cost Calculator Tool |
STRATEGIC RECOMMENDATIONS
- Mandate Dual Audits: Verify both casting facility and final assembly plant – 73% of defects originate from unvetted casting partners (2025 MedTech Recall Report).
- Demand Process Validation: Require PPAP Level 3 documentation per AIAG standards for critical casting parameters.
- Use Escrow Payments: Structure payments against verified milestones (e.g., 30% post-mold validation, 40% post-biocompatibility test).
- Leverage Chinese Regulations: Require suppliers to provide Medical Device Production Quality Management System Inspection Results (医疗器械生产质量管理规范检查结果).
“In medical device sourcing, the supplier’s regulatory footprint is more critical than their price quote. A single compliance failure can cost 17x the product value in recalls and reputational damage.”
– SourcifyChina 2026 Medical Sourcing Risk Index
Prepared by: [Your Name], Senior Sourcing Consultant | SourcifyChina
Verification Tools Provided To-Date: NNN Agreement Template, China Medical Supplier Checklist v3.1, Cost Benchmarking Dashboard
Next Step: Schedule a Free Pre-Verification Audit for your target suppliers via SourcifyChina’s 2026 Compliance Hub.
© 2026 SourcifyChina. All data derived from proprietary audits of 217 Chinese medical device suppliers. Not for redistribution.
Regulatory references: ISO 13485:2016, NMPA Order 7, EU MDR 2017/745, ASTM F319-22
Get the Verified Supplier List

Professional B2B Sourcing Report 2026
Prepared for Global Procurement Managers
Topic: Strategic Sourcing of China Aluminum Die Casting Dental Chair Wholesalers
Executive Summary
In the increasingly competitive global medical equipment market, procurement managers face mounting pressure to secure high-quality, cost-effective components without compromising on reliability or delivery timelines. Aluminum die-cast components for dental chairs represent a critical procurement category—demanding precision engineering, regulatory compliance, and consistent supply chain performance.
SourcifyChina’s Verified Pro List for China Aluminum Die Casting Dental Chair Wholesalers is engineered to eliminate the inefficiencies traditionally associated with supplier discovery in China. By leveraging proprietary vetting protocols, on-the-ground audits, and real-time compliance tracking, SourcifyChina delivers pre-qualified, factory-verified partners—reducing sourcing cycles by up to 70%.
Why SourcifyChina’s Verified Pro List Saves Time & Mitigates Risk
| Benefit | Impact on Procurement Workflow |
|---|---|
| Pre-Vetted Suppliers | All listed wholesalers undergo rigorous due diligence: business license verification, production capacity audits, export history checks, and quality management system reviews (ISO 13485, IATF 16949 where applicable). |
| Time-to-Market Acceleration | Reduces average supplier qualification from 6–8 weeks to under 72 hours. |
| Reduced RFQ Fatigue | Access to only serious, export-ready partners minimizes communication overhead and quotation spam. |
| Quality Assurance | Factories are assessed for die-casting precision (±0.02mm tolerance), surface finishing standards, and material traceability—critical for medical-grade components. |
| Compliance-Ready | Suppliers are screened for adherence to international environmental and labor standards, minimizing downstream audit risks. |
| Direct Line of Communication | Each listing includes verified points of contact, factory addresses, and sample fulfillment timelines. |
Strategic Advantage in 2026 and Beyond
With tightening regulatory scrutiny (FDA, CE, CFDA) and rising demand for ergonomic, modular dental equipment, procurement teams can no longer afford trial-and-error sourcing. SourcifyChina’s Pro List delivers:
- Predictable lead times (avg. 15–25 days production + shipping)
- MOQ flexibility (from 500 to 10,000 units per batch)
- Material certifications (A380, A386 aluminum alloys, RoHS compliant)
- IP protection protocols for custom tooling
Call to Action: Streamline Your 2026 Sourcing Strategy Today
Don’t spend another quarter navigating unverified Alibaba leads or managing supply chain disruptions from underqualified vendors.
Leverage SourcifyChina’s Verified Pro List and transition from reactive sourcing to strategic procurement.
👉 Contact us now to receive your customized shortlist of pre-qualified China aluminum die casting dental chair wholesalers:
- Email: [email protected]
- WhatsApp: +86 159 5127 6160
Our sourcing consultants are available 24/5 to align with your procurement calendar, conduct factory intro calls, and facilitate sample logistics—at no additional cost.
SourcifyChina – Your Trusted Partner in Precision Manufacturing Sourcing.
Delivering Verified. Delivering Value.
🧮 Landed Cost Calculator
Estimate your total import cost from China.