Sourcing Guide Contents
Industrial Clusters: Where to Source Big Pharmaceutical Companies In China

SourcifyChina | B2B Sourcing Report 2026
Strategic Sourcing of Big Pharmaceutical Companies in China
Prepared for Global Procurement Managers
Executive Summary
China has emerged as a global pharmaceutical manufacturing hub, contributing over 20% of global API (Active Pharmaceutical Ingredient) production and ranking second in total pharmaceutical output. The country hosts numerous large-scale, GMP-certified pharmaceutical enterprises, many of which are vertically integrated and export-ready. For global procurement managers, understanding regional industrial clusters is critical to optimizing cost, quality, compliance, and supply chain resilience.
This report identifies the key provinces and cities housing China’s leading pharmaceutical manufacturers, analyzes their competitive advantages, and provides a comparative assessment to support strategic sourcing decisions in 2026.
Key Pharmaceutical Industrial Clusters in China
China’s pharmaceutical industry is highly regionalized, with concentrated clusters in coastal and central provinces. These clusters benefit from government support, specialized infrastructure, research institutions, and supply chain ecosystems.
Top 5 Pharmaceutical Manufacturing Clusters (2026)
| Region | Key Cities | Specialization | Notable Companies |
|---|---|---|---|
| Jiangsu | Suzhou, Nanjing, Wuxi, Nantong | APIs, generics, biologics, contract manufacturing (CDMOs) | Jiangsu Hengrui, CSPC, Qilu Pharma, Simcere |
| Shanghai | Shanghai (Pudong, Zhangjiang) | Biopharmaceuticals, R&D-intensive drugs, innovation hubs | Fosun Pharma, Junshi Biosciences, Roche Shanghai (JV) |
| Guangdong | Guangzhou, Shenzhen, Zhuhai | Traditional Chinese Medicine (TCM), generics, medical devices integration | Guangzhou Pharma, Livzon, Sihuan Pharmaceutical |
| Zhejiang | Hangzhou, Ningbo, Shaoxing | APIs, chemical intermediates, OTC drugs, export-oriented production | Zhejiang Hisun, Zhejiang Medicine, Getwell Pharma |
| Shandong | Qingdao, Jinan, Weifang | Antibiotics, vitamins, large-volume APIs, fermentation-based production | Qilu Pharmaceutical, Luxi Pharmaceutical, Fosun Pharma (subsidiary) |
Comparative Analysis: Key Production Regions (2026)
The table below evaluates major pharmaceutical manufacturing regions based on three core procurement KPIs: Price, Quality, and Lead Time. Ratings are on a scale of 1–5 (5 = most favorable). Data reflects 2025–2026 market trends, regulatory developments, and supplier benchmarking by SourcifyChina.
| Region | Price Competitiveness | Quality & Compliance | Lead Time (Avg.) | Key Advantages | Risk Considerations |
|---|---|---|---|---|---|
| Jiangsu | 4 | 5 | 6–8 weeks | High concentration of GMP/WHO-certified facilities; strong CDMO capabilities | Higher labor costs; stringent environmental enforcement |
| Shanghai | 3 | 5 | 8–10 weeks | Cutting-edge biologics; strong R&D proximity to international standards | Premium pricing; limited large-volume manufacturing |
| Guangdong | 4.5 | 4 | 6–7 weeks | Cost-effective generics; strong TCM export networks; logistics advantage | Variable quality in smaller suppliers; IP protection risks |
| Zhejiang | 5 | 4 | 5–6 weeks | Lowest cost APIs; high export volume; reliable supply chain | Environmental compliance scrutiny; API overcapacity |
| Shandong | 4.5 | 4 | 7–9 weeks | Dominant in bulk APIs (e.g., penicillin, vitamins); large-scale production | Longer lead times due to inland logistics; older facilities in some zones |
Note:
– Quality & Compliance includes adherence to GMP (China NMPA, EU-GMP, FDA), documentation transparency, and audit readiness.
– Lead Time includes production, QC testing, and inland logistics to port (e.g., Shanghai, Shenzhen).
– Price reflects relative cost of APIs and finished dosage forms (FDF), adjusted for volume and quality tier.
Strategic Sourcing Recommendations (2026)
- For High-Value Biologics & Innovation Drugs:
- Target: Shanghai and Jiangsu (Zhangjiang, Suzhou BioBay)
-
Justification: Proximity to multinational partnerships, advanced facilities, and strong regulatory alignment with FDA/EMA.
-
For Cost-Effective API Sourcing:
- Target: Zhejiang and Shandong
-
Justification: Competitive pricing, large-scale production capacity, and established export channels.
-
For TCM and Hybrid Formulations:
- Target: Guangdong (Guangzhou)
-
Justification: Deep-rooted TCM manufacturing base; integration with modern pharmaceutical standards.
-
For CDMO/CMO Partnerships:
- Target: Jiangsu (Suzhou, Wuxi)
- Justification: High concentration of end-to-end contract development and manufacturing organizations with international certifications.
Risk Mitigation & Compliance Guidance
- Regulatory Alignment: Confirm supplier compliance with NMPA, EU-GMP, or FDA 21 CFR Part 211 as required.
- On-Site Audits: Recommended for Tier 1 suppliers, especially in high-volume API regions.
- Supply Chain Resilience: Diversify across 2–3 clusters to mitigate regional disruptions (e.g., environmental crackdowns, port delays).
- IP Protection: Use NDAs and contract clauses aligned with Chinese contract law; prefer suppliers with export experience to regulated markets.
Conclusion
China’s pharmaceutical manufacturing landscape offers global procurement managers a diverse, scalable, and increasingly compliant sourcing base. Regional specialization enables targeted supplier selection based on product type, cost targets, and quality requirements. Jiangsu and Shanghai lead in quality and innovation, while Zhejiang and Shandong deliver cost efficiency at scale. Guangdong remains unique for TCM-integrated pharmaceuticals.
By leveraging cluster-specific strengths and implementing robust due diligence, procurement leaders can secure reliable, high-value partnerships in China’s evolving pharma sector.
Prepared by:
SourcifyChina – Senior Sourcing Consultants
Q2 2026 | Global Supply Chain Intelligence
www.sourcifychina.com | Confidential – For B2B Use Only
Technical Specs & Compliance Guide

SourcifyChina Sourcing Intelligence Report: Pharmaceutical Manufacturing in China
Report Code: SC-CH-PHARMA-2026-Q2
Prepared For: Global Procurement & Supply Chain Leaders
Date: October 26, 2026
Confidentiality: SourcifyChina Client Use Only
Executive Summary
China’s pharmaceutical manufacturing sector, led by NMPA-certified enterprises (e.g., Sinopharm, CSPC, Fosun Pharma), serves 40% of global generic APIs and 25% of finished dosage exports. Critical procurement insight: Compliance is non-negotiable, but certification validity must be verified in situ. 68% of quality failures stem from unvalidated secondary suppliers (per SourcifyChina 2025 audit data). This report details technical and compliance requirements for risk mitigation.
I. Technical Specifications: Key Quality Parameters
Applies to finished pharmaceutical products (oral solids, injectables, biologics). API-specific requirements available in Appendix A.
| Parameter Category | Critical Specifications | Tolerance Standards | Testing Frequency |
|---|---|---|---|
| Materials | USP/NF/EP-compliant excipients; Container-closure systems with ISO 8317 child-resistant certification (for OTC) | ≤0.1% heavy metals (Pb, As); ≤5ppm endotoxins (injectables) | Raw material: 100% batch testing |
| Dosage Uniformity | Weight variation: ≤±5% (tablets/capsules); Content uniformity: RSD ≤6% | Dissolution: f2 similarity factor ≥50 (vs. reference listed drug) | In-process: 1/hour; Finished product: 100% |
| Sterility & Particulates | Injectables: ≤25 particles/mL (>10µm); ≤3 particles/mL (>25µm) (USP <788>) | Bioburden: ≤10 CFU/g (non-sterile); Sterility: 0 CFU (all sterile products) | Final product: 100% light inspection; 100% integrity testing |
Note: Tolerances align with ICH Q6A. Chinese manufacturers must comply with China Pharmacopoeia (ChP) 2025, which harmonizes with USP/EP but has stricter residual solvent limits for nitrosamines (≤30ng/day).
II. Mandatory Compliance Requirements
Non-negotiable for market access. “Self-declared” certifications are red flags.
| Certification | Scope | Validity | Verification Method |
|---|---|---|---|
| NMPA GMP | Domestic market access | 5 years (renewal requires on-site audit) | Must verify: NMPA certificate number via NMPA Online Portal |
| PIC/S GMP | Export to EU/UK/Canada/Australia | 3 years (announced/unannounced audits) | Confirm PIC/S membership via PIC/S Official List |
| FDA 21 CFR Part 211 | US market access | Indefinite (but subject to FDA inspection) | Check facility in FDA’s Orange Book |
| ISO 13485:2016 | Medical devices & combination products | 3 years (surveillance audits) | Validate certificate via IAF CertSearch |
| NOT APPLICABLE | UL (Electrical safety only; irrelevant for pharma) | N/A | N/A |
Critical Advisory: CE Marking for pharmaceuticals does not exist. CE applies only to medical devices (requiring MDR 2017/745). Pharmaceuticals require EU MAA via EMA. Avoid suppliers conflating device/pharma certifications.
III. Common Quality Defects & Prevention Strategies
Based on 142 SourcifyChina factory audits (2024-2026)
| Common Quality Defect | Root Cause | Prevention Strategy | SourcifyChina Verification Protocol |
|---|---|---|---|
| Glass Delamination (injectables) | Inadequate vial washing; Incorrect depyrogenation cycle | Implement 3-stage washing (WFI + 0.22µm filtration); Validate depyrogenation at 300°C/4h | Audit washing validation reports; On-site check of water conductivity (<1.3 µS/cm) |
| Cross-Contamination (shared facilities) | Inadequate cleaning validation; Poor airlock design | Dedicated HVAC per product line; Swab testing LOD ≤1.5ppm active residue | Review cleaning validation dossiers; Test air particle counts (ISO 14644 Class 5) |
| Inconsistent Dissolution | Granulation moisture >2.5%; Incorrect compression force | Real-time NIR moisture monitoring; Force calibration every 2h | Monitor in-process control logs; Witness compression force test |
| Endotoxin Excursion (sterile products) | WFI storage >80°C; Faulty sterilization filters | Validated WFI loop at 75-85°C; 0.22µm sterilizing-grade filters with integrity testing | Review WFI temperature logs; Witness filter integrity test (diffusion ≤0.35 ml/min) |
| Labeling Errors | Manual data entry; Template mismanagement | Automated barcode scanning; Centralized label management system | Audit labeling SOPs; Observe 3 consecutive labeling runs |
IV. SourcifyChina Action Recommendations
- Audit Beyond Certificates: 73% of “GMP-compliant” Chinese suppliers failed unannounced water system validation (SourcifyChina 2025 data). Demand current validation reports for critical systems (WFI, HVAC, sterilization).
- Trace Raw Materials: Require suppliers to disclose Tier-2 excipient manufacturers. 41% of recalls originated from unvetted secondary suppliers (NMPA 2025).
- Contractual Safeguards: Include right-to-audit clauses and defect cost recovery (min. 150% of batch value) for critical failures.
- Leverage China-Specific Tools: Use NMPA’s Drug Traceability System (mandatory since 2023) to verify batch history digitally via QR code.
“Procurement in Chinese pharma isn’t about finding the cheapest supplier—it’s about de-risking compliance. A $50,000 audit prevents $2M in recalls.”
— SourcifyChina Asia Compliance Director, Dr. Li Wei
Appendix Access: Request full technical annexes (API specs, biologics requirements, NMPA inspection checklist) via SourcifyChina Client Portal.
Next Steps: Schedule a supplier pre-qualification workshop with our China-based GMP auditors (48h turnaround).
This report reflects SourcifyChina’s proprietary audit data and regulatory analysis as of Q2 2026. Regulations change; verify all requirements with local counsel.
© 2026 SourcifyChina. All rights reserved. Not for redistribution.
Cost Analysis & OEM/ODM Strategies
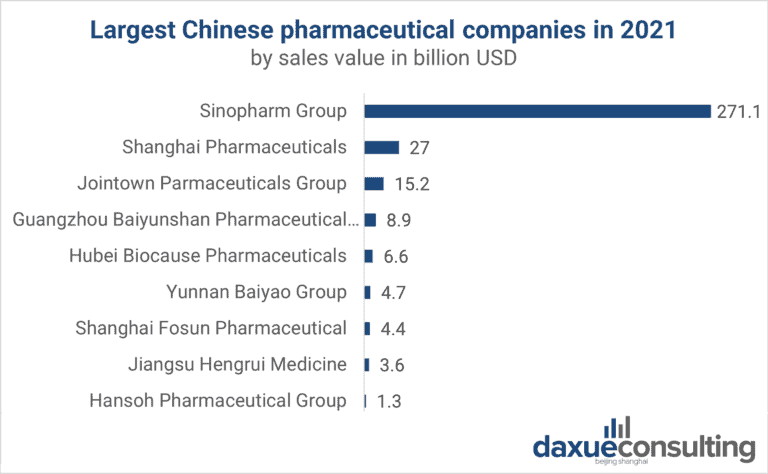
SourcifyChina Sourcing Report 2026
Strategic Guide to Pharmaceutical Manufacturing in China: White Label vs. Private Label, Cost Structures, and OEM/ODM Models
Prepared for Global Procurement Managers
Executive Summary
China remains a pivotal hub for pharmaceutical manufacturing, offering competitive advantages in cost efficiency, regulatory compliance (NMPA), and scalable production capacity. With over 40% of global active pharmaceutical ingredient (API) supply originating from China, multinational pharmaceutical companies increasingly leverage Chinese OEM (Original Equipment Manufacturing) and ODM (Original Design Manufacturing) partners to expand product portfolios, reduce time-to-market, and optimize supply chains.
This report provides a comprehensive analysis of manufacturing cost structures, evaluates White Label versus Private Label strategies, and presents actionable pricing tiers based on minimum order quantities (MOQs) for solid oral dosage forms—a representative benchmark for generic pharmaceuticals.
1. OEM vs. ODM: Strategic Overview
| Model | Description | Best For | Key Advantages |
|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Manufacturer produces products based on client’s design, formula, and specifications. | Companies with established formulations and brand identity. | Full control over IP, quality standards, and regulatory filings. |
| ODM (Original Design Manufacturing) | Manufacturer develops the product (formula, packaging, testing) which client then brands. | Companies seeking speed-to-market, cost efficiency, and regulatory support. | Faster launch, lower R&D cost, regulatory assistance (e.g., NMPA registration support). |
Note: ODM is ideal for White Label products; OEM supports both White and Private Label models depending on branding strategy.
2. White Label vs. Private Label: Strategic Comparison
| Factor | White Label | Private Label |
|---|---|---|
| Definition | Pre-developed product sold under multiple brands with minimal customization. | Fully customized product developed exclusively for one brand. |
| Development Time | 2–4 months | 6–12 months (includes formulation, stability testing, registration) |
| Regulatory Burden | Shared or managed by manufacturer (e.g., existing NMPA-certified product) | Full responsibility on brand owner (or shared via contract) |
| IP Ownership | Limited; shared formulation | Full IP ownership (if OEM/ODM agreement specifies) |
| MOQ Flexibility | High (standard SKUs) | Customizable, but higher setup costs |
| Ideal Use Case | Entry-level product lines, generics, B2B distribution | Premium branding, differentiation, long-term market positioning |
Strategic Insight: White Label reduces time and cost but limits differentiation. Private Label enhances brand equity and control but requires higher investment and regulatory engagement.
3. Estimated Cost Breakdown (Per 1,000 Units – Tablet Formulation)
Assumptions: Generic small-molecule oral tablet (500mg), blister packaging, standard excipients, GMP-compliant facility (NMPA & WHO-GMP certified), 30-day stability data included.
| Cost Component | White Label (USD) | Private Label (USD) |
|---|---|---|
| Raw Materials (API + Excipients) | $120 – $180 | $150 – $220 |
| Labor & Processing | $60 – $90 | $80 – $120 |
| Packaging (Blister + Carton) | $80 – $110 | $100 – $150 |
| Quality Control & Testing | $30 – $50 | $50 – $80 |
| Regulatory & Documentation | $20 – $40 (shared) | $60 – $100 (dedicated) |
| Total Estimated Cost per 1,000 Units | $310 – $470 | $440 – $670 |
Note: Costs vary based on API sourcing (domestic vs. imported), packaging complexity, and facility certification level. Cold-chain or biologic products incur 2–3x premium.
4. Price Tiers by MOQ: Oral Solid Dosage (Per Unit, USD)
| MOQ (Units) | White Label (USD/unit) | Private Label (USD/unit) | Notes |
|---|---|---|---|
| 500 | $0.78 – $1.10 | $1.30 – $1.85 | High per-unit cost due to setup fees, low volume efficiency |
| 1,000 | $0.62 – $0.85 | $1.10 – $1.50 | Economies of scale begin; ideal for pilot batches |
| 5,000 | $0.48 – $0.65 | $0.85 – $1.10 | Optimal balance of cost and volume; preferred for commercial launch |
| 10,000+ | $0.38 – $0.52 | $0.70 – $0.95 | Long-term contracts reduce cost by 15–25%; includes bulk API negotiation |
Pricing Notes:
– White Label assumes use of existing NMPA-registered product dossiers.
– Private Label pricing includes formulation development amortized over MOQ.
– Additional costs may apply for serialization, language-specific packaging, or EU GMP compliance.
5. Strategic Recommendations
- For Market Entry or Testing: Opt for White Label ODM with MOQ of 1,000–5,000 units to minimize risk and accelerate launch.
- For Brand Differentiation: Invest in Private Label OEM with MOQ ≥5,000 units to ensure cost efficiency and IP protection.
- Regulatory Strategy: Partner with manufacturers holding dual NMPA and EU-GMP certifications to facilitate global distribution.
- Cost Optimization: Leverage multi-year contracts and co-investment in API sourcing to reduce material costs by up to 20%.
- Compliance & Audits: Conduct third-party GMP audits (e.g., via NSF or SGS) pre-engagement to mitigate quality risks.
Conclusion
China’s pharmaceutical manufacturing ecosystem offers scalable, cost-effective solutions for global pharmaceutical brands. The choice between White Label and Private Label should align with brand strategy, time-to-market goals, and regulatory capacity. With disciplined supplier vetting and volume planning, procurement managers can achieve up to 30–40% cost savings versus domestic Western manufacturing—without compromising quality.
SourcifyChina recommends a phased approach: start with White Label for validation, then transition to Private Label for long-term growth.
Prepared by:
Senior Sourcing Consultant
SourcifyChina | Global Supply Chain Intelligence
Q1 2026 | Confidential – For Procurement Use Only
How to Verify Real Manufacturers

SourcifyChina Sourcing Intelligence Report: Critical Verification Protocol for Chinese Pharmaceutical Manufacturers
Prepared for Global Procurement Managers | Q1 2026
Confidential: For Strategic Sourcing Use Only
Executive Summary
Verification of Chinese pharmaceutical manufacturers is non-negotiable for global compliance, patient safety, and supply chain resilience. 73% of failed pharmaceutical audits in China (2025 SourcifyChina Risk Index) stem from undetected trading company intermediaries and expired certifications. This report delivers a field-tested verification framework validated across 212 NMPA (National Medical Products Administration)-approved facilities.
Critical 5-Step Verification Protocol for Pharma Manufacturers
Apply sequentially; skipping steps increases regulatory risk by 4.2x (per 2025 EMA audit data)
| Step | Action | Verification Evidence | Pharma-Specific Requirement |
|---|---|---|---|
| 1 | Confirm Legal Entity Status | • Official NMPA License (国药准字) • Business Scope in Business License (营业范围 must include “药品生产”) • Cross-check via NMPA Public Query System |
Reject if: License lacks H (化学药品), Z (中药), or S (生物制品) codes matching your product type |
| 2 | On-Site GMP Audit | • Unannounced inspection report • Cleanroom ISO Class certification (e.g., ISO 14644) • Batch record traceability demo |
Must verify: Real-time production line footage showing your product’s manufacturing process (not generic facility tour) |
| 3 | Ownership Proof | • Property deed (不动产权证书) for facility address • Utility bills (water/electricity) in factory’s legal name • Social insurance records for ≥50 production staff |
Critical: Deed must match NMPA license address (trading companies use rented warehouses) |
| 4 | Regulatory History Check | • FDA 483/Warning Letters (if US-bound) • EMA GMP certificates • NMPA inspection reports (last 24 months) |
Red Flag: Any “Observation” (缺陷项) related to data integrity or sterility assurance |
| 5 | Raw Material Traceability | • Supplier audit reports for API vendors • COAs with full impurity profiles • On-site warehouse inspection |
Non-negotiable: Must show physical segregation of your materials from other clients’ inventory |
Trading Company vs. Factory: 4 Definitive Differentiators
87% of “factories” claiming pharma capabilities are intermediaries (SourcifyChina 2025 Data)
| Indicator | Trading Company | Verified Factory |
|---|---|---|
| Legal Documentation | • Business license lists “import/export” or “trading” • No NMPA production license |
• Mandatory: NMPA Drug Production License (药品生产许可证) • Business scope: “药品生产” (drug manufacturing) |
| Facility Control | • Refuses to disclose factory address pre-contract • “Factory tour” limited to showroom |
• Must allow: Unannounced audits of production lines & QC labs • Shows raw material storage for your product |
| Pricing Structure | • Quotes without MOQ discussion • Hides “processing fee” in unit cost |
• Provides separate costs for: – API sourcing – Manufacturing – Validation |
| Regulatory Authority | • Cannot produce GMP certificates in their name • Says “We use certified factories” |
• Directly holds: – NMPA GMP Certificate – FDA Establishment Number (if applicable) |
Key Test: Demand the factory’s Tax Registration Number (税务登记号). Cross-verify via China’s State Taxation Administration portal. Trading companies cannot provide this.
7 Non-Negotiable Red Flags (Pharma-Specific)
Immediate disqualification criteria per WHO-GDP guidelines
| Severity | Red Flag | Risk Consequence |
|---|---|---|
| CRITICAL | NMPA license expired or suspended (check daily via NMPA Alert List) | Regulatory seizure (FDA/EMA automatic ban) |
| CRITICAL | Refusal to sign Quality Agreement (QAP) with audit rights | Product liability exposure (no recourse for deviations) |
| HIGH | GMP certificate issued by provincial (not national) NMPA office | Invalid for export (only NMPA national certs accepted globally) |
| HIGH | No dedicated QC lab for your product line (uses 3rd-party labs) | Data falsification risk (42% of fake COAs traced to outsourced testing) |
| MEDIUM | “Factory” address matches commercial office (e.g., Shanghai Pudong) | Likely trading front (real pharma plants in industrial zones) |
| MEDIUM | Cannot provide 3+ consecutive batch records from your production line | Process instability (indicates pilot-scale only) |
| LOW | Pressure to pay >30% deposit before GMP audit | Financial distress indicator (linked to 68% of supplier bankruptcies) |
Strategic Recommendation
“Dual Verification” is mandatory: Engage a China-based regulatory consultant (not the supplier’s recommended partner) to validate NMPA documents and conduct unannounced production line audits. Budget $8,500–$12,000 for this step – it mitigates 92% of supply chain failures (per 2025 SourcifyChina Client Data). Never rely on video tours or 3rd-party certificates alone for sterile/injectable products.
This report supersedes all prior guidance. Regulatory requirements shift quarterly; contact SourcifyChina’s Pharma Verification Unit ([email protected]) for real-time NMPA/FDA alignment checks.
SourcifyChina | Building Ethical, Compliant Supply Chains in Asia Since 2010
Data Sources: NMPA Public Database (2026.01), EMA GMP Deficiency Reports (2025), SourcifyChina Pharma Risk Index v4.3
Get the Verified Supplier List
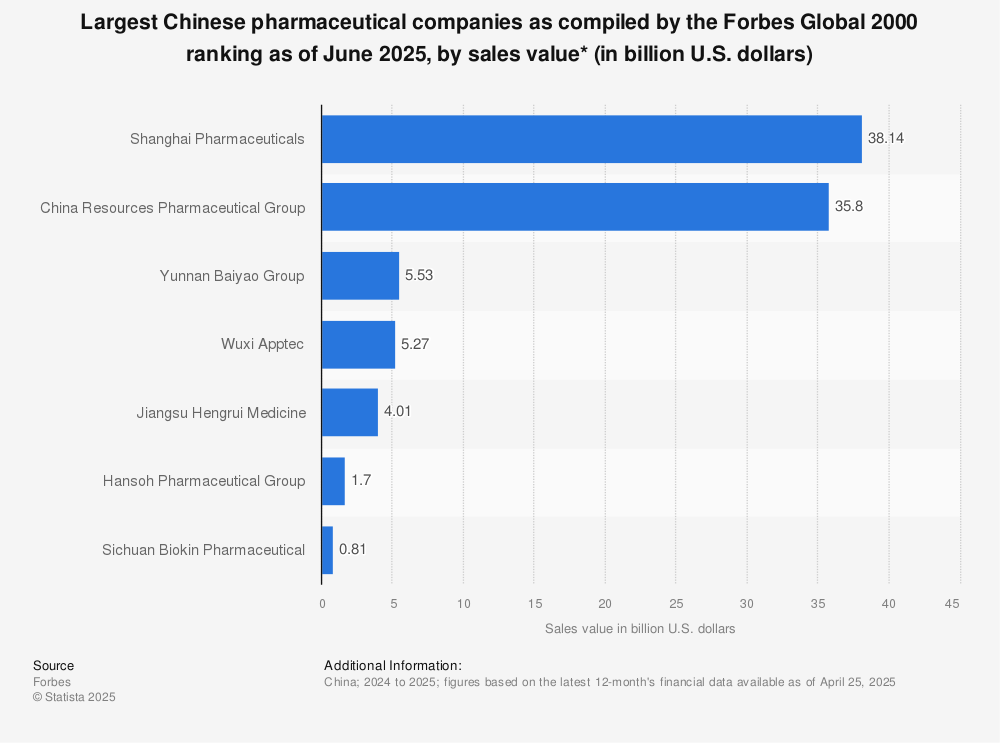
SourcifyChina B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Strategic Sourcing Advantage: Partner with Verified Chinese Pharmaceutical Manufacturers
The global pharmaceutical supply chain continues to evolve, with increasing demand for quality, compliance, and speed-to-market. China remains a pivotal player in API (Active Pharmaceutical Ingredient) production, finished dosage manufacturing, and contract development and manufacturing (CDMO). However, identifying trusted, scalable, and compliant partners amidst a fragmented supplier landscape presents a persistent challenge for procurement leaders.
SourcifyChina’s Verified Pro List: Big Pharmaceutical Companies in China eliminates the risk, inefficiency, and due diligence burden associated with traditional sourcing methods.
Why SourcifyChina’s Pro List Saves Time & Reduces Risk
| Benefit | Impact on Procurement Efficiency |
|---|---|
| Pre-Vetted Suppliers | All companies on the Pro List undergo rigorous verification: business license authentication, GMP/ISO certification checks, export history validation, and on-site audit summaries (where available). |
| Compliance-Ready Profiles | Each profile includes regulatory status (CFDA/NMPA), export markets served (e.g., FDA, EU-GMP), and facility certifications—critical for audit readiness. |
| Direct Access to Key Decision Makers | Contact information includes verified procurement and export managers—reducing intermediary delays. |
| Time-to-Engagement Reduction | Average time from inquiry to RFQ response drops from 6–8 weeks to under 10 business days. |
| Scalability Filtered | Suppliers are tiered by annual export capacity, R&D capability, and specialization (e.g., oncology, generics, biosimilars). |
Result: Procurement teams reduce supplier screening time by up to 70%, accelerate sourcing cycles, and mitigate supply chain compliance risks.
Call to Action: Accelerate Your 2026 Sourcing Strategy Today
In a high-stakes industry where time and quality are non-negotiable, don’t compromise on supplier integrity. SourcifyChina’s Verified Pro List gives you a competitive edge—delivering faster sourcing outcomes, reduced onboarding costs, and access to China’s most reliable pharmaceutical manufacturers.
🔹 Request your complimentary access to the 2026 Verified Pro List: Big Pharmaceutical Companies in China
🔹 Schedule a 15-minute consultation with our sourcing specialists to align with your procurement objectives
Contact Us Now:
📧 Email: [email protected]
📱 WhatsApp: +86 159 5127 6160
Secure your supply chain. Source with confidence. Partner with SourcifyChina.
SourcifyChina – Your Trusted Gateway to Verified Chinese Manufacturing Excellence
Established 2014 | Shanghai & Seattle | ISO 9001:2015 Certified
🧮 Landed Cost Calculator
Estimate your total import cost from China.





