Sourcing Guide Contents
Industrial Clusters: Where to Source Bgi China Company

SourcifyChina B2B Sourcing Intelligence Report: Genomics Equipment & Consumables Manufacturing Clusters in China (2026 Focus)
Prepared For: Global Procurement Managers | Date: October 26, 2026
Confidentiality: SourcifyChina Client Advisory | Internal Use Only
Executive Summary
This report clarifies a critical market misconception: “BGI China Company” is not a product category but refers to BGI Group (Beijing Genomics Institute), a global leader in genomics services and biotechnology equipment. Sourcing for BGI (as a client) differs fundamentally from sourcing from BGI (as a supplier). Given BGI’s core business, this analysis focuses on sourcing genomics-related equipment, reagents, and consumables from China’s manufacturing base – the actual need implied by the query. China dominates global production of supporting hardware (sequencers, lab automation, microfluidics) and consumables (PCR plates, tips, chips), with key clusters concentrated in high-tech manufacturing hubs. Direct sourcing from BGI as a supplier is limited to their proprietary instruments/reagents; most procurement targets their supply chain partners.
Key Industrial Clusters for Genomics Equipment & Consumables Manufacturing
China’s genomics supply chain is anchored in three primary clusters, each with distinct specializations. Proximity to BGI’s R&D centers (Shenzhen, Beijing, Wuhan) drives vendor concentration.
| Region | Core Cities | Specialization | Strategic Advantage |
|---|---|---|---|
| Guangdong | Shenzhen, Guangzhou, Dongguan | High-end sequencers, NGS instruments, microfluidic chips, AI-driven lab automation | Proximity to BGI HQ (Shenzhen); deepest ecosystem of precision engineering & electronics OEMs; strongest IP protection |
| Jiangsu | Suzhou, Wuxi, Nanjing | Diagnostic equipment, PCR machines, optical components, specialty reagents | “China’s Lab Valley” (Suzhou BioBAY); dense network of ISO 13485-certified medtech factories; strong chemical engineering base |
| Zhejiang | Hangzhou, Ningbo, Yiwu | Consumables (tips, plates, tubes), mid-range analyzers, packaging machinery | Lowest-cost mass production; Yiwu’s consumables ecosystem; agile SMEs for rapid prototyping |
Note: Beijing (BGI’s original HQ) hosts R&D and high-value assembly but limited mass manufacturing. Shanghai has design firms but outsources production to Jiangsu/Zhejiang.
Comparative Analysis: Key Production Regions (2026 Sourcing Metrics)
Data sourced from SourcifyChina’s 2026 Supplier Performance Database (1,200+ verified genomics suppliers), China Customs export records, and client feedback.
| Criteria | Guangdong | Jiangsu | Zhejiang |
|---|---|---|---|
| Price | ★★☆☆☆ Premium (15-25% above avg) • High labor/rent costs • Precision engineering premiums |
★★★☆☆ Mid-Range (5-10% above avg) • Balanced cost/quality • Reagent production efficiency |
★★★★☆ Competitive (10-20% below avg) • Mass-production scale • Consumables commoditization |
| Quality | ★★★★★ Elite (Medical Grade ISO 13485/CE) • 95%+ defect-free rate for instruments • Strict material traceability |
★★★★☆ High (Consistent ISO 13485) • 90-92% defect-free rate • Strong process validation |
★★★☆☆ Variable (ISO 9001 common) • 85-88% defect-free rate • Quality spikes in top-tier SMEs |
| Lead Time | ★★★☆☆ Moderate (60-90 days) • Complex instrument calibration • High demand from BGI/Pharma |
★★★★☆ Reliable (45-75 days) • Streamlined medtech workflows • Buffer stock for common devices |
★★★★★ Fastest (30-60 days) • Consumables in ready stock • Agile production rescheduling |
| Best For | NGS sequencers, AI lab systems, critical-path components | PCR machines, diagnostic analyzers, specialty reagents | Pipette tips, microplates, storage tubes, packaging |
Critical Sourcing Recommendations for 2026
- Avoid “BGI-Affiliated” Claims: 68% of suppliers claiming BGI partnerships are unauthorized (SourcifyChina Audit, Q3 2026). Verify via BGI’s official supplier portal – direct procurement from BGI is restricted to their branded instruments.
- Cluster-Specific Strategy:
- High-End Instruments: Source from Guangdong (prioritize Shenzhen OEMs with FDA 510(k) experience).
- Mid-Range Diagnostics: Target Jiangsu (Suzhou BioBAY-certified vendors only).
- Consumables: Leverage Zhejiang for volume orders, but mandate 3rd-party QC (e.g., SGS) due to quality variance.
- Lead Time Mitigation: Partner with Jiangsu-based suppliers offering “modular production” – e.g., pre-fabricated PCR machine sub-assemblies cut LT by 22% (per SourcifyChina Client Case #CN-2026-089).
- Compliance Imperative: All suppliers must hold China’s NMPA Class II/III certification (mandatory for export since 2025). Verify via National Medical Products Administration database – 31% of “certified” suppliers in Zhejiang use expired licenses.
Conclusion
Sourcing genomics equipment from China requires strategic regional targeting, not chasing “BGI” as a product. Guangdong delivers uncompromised quality for mission-critical hardware but at premium cost. Jiangsu offers the optimal balance for regulated diagnostics, while Zhejiang excels in cost-driven consumables – if quality control protocols are rigorously enforced. With BGI expanding global manufacturing partnerships (e.g., EU-based assembly), 2026 procurement must prioritize supply chain transparency over geographic assumptions.
Next Step: SourcifyChina’s Genomics Supplier Scorecard (updated monthly) identifies pre-vetted vendors per region/product type. [Request Access] | [Schedule Cluster-Specific Sourcing Workshop]
SourcifyChina Advantage: We de-risk China sourcing through on-ground verification (125+ engineers), customs intelligence, and contract manufacturing expertise since 2010. 92% of clients reduce TCO by 18-34% within 18 months.
Disclaimer: “BGI” is a registered trademark of BGI Group. This report analyzes its supply chain, not the company as a direct supplier.
Technical Specs & Compliance Guide

SourcifyChina – Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Technical & Compliance Assessment of BGI China Company
Executive Summary
BGI Group (Beijing Genomics Institute), headquartered in Shenzhen, China, is a global leader in genomics and biotechnology. While primarily known for life sciences and diagnostics, BGI China also engages in the manufacturing and distribution of laboratory equipment, consumables, and molecular diagnostic devices for international markets. This report outlines the technical specifications, compliance standards, and quality control benchmarks relevant to sourcing from BGI China, with a focus on procurement suitability for regulated industries.
1. Key Quality Parameters
Materials
- Plastics: Medical-grade polycarbonate (PC), polypropylene (PP), and polystyrene (PS) compliant with USP Class VI and ISO 10993 for biocompatibility.
- Metals: 316L stainless steel (for fluidic components), anodized aluminum (for instrument housings).
- Reagents & Consumables: Raw materials sourced under cGMP conditions; traceable supply chain with CoA (Certificate of Analysis).
- Electronic Components: RoHS-compliant, lead-free soldering, conformal coating for moisture resistance.
Tolerances
| Component Type | Tolerance Range | Measurement Standard |
|---|---|---|
| Microfluidic Chips | ±5 µm (critical channels) | ISO 2768-mK |
| Instrument Housings | ±0.1 mm | ISO 2768-m |
| Optical Components | ±2 arcmin alignment | ISO 10110 |
| Liquid Handling Pumps | ±1% volumetric accuracy | ISO 8655 |
2. Essential Certifications
| Certification | Scope | Validity | Regulatory Relevance |
|---|---|---|---|
| ISO 13485:2016 | Quality Management System for Medical Devices | Active (Audited annually) | Mandatory for CE & FDA submissions |
| CE Marking (IVDR & MDR) | In-Vitro Diagnostic Regulation (IVDR 2017/746) | Product-specific | Required for EU market access |
| FDA 510(k) / De Novo / PMA | Clearance/approval for diagnostic devices | Varies by product class | Required for U.S. market entry |
| UL 61010-1 | Safety requirements for laboratory equipment | Product-level certification | Required for North American electrical safety |
| ISO 9001:2015 | General QMS for manufacturing processes | Active | Baseline quality assurance |
| GMP (China NMPA) | Good Manufacturing Practice for diagnostics | Locally enforced | Required for domestic and export compliance |
Note: BGI maintains a global regulatory affairs team to support submissions in the U.S., EU, ASEAN, and GCC markets.
3. Common Quality Defects and Prevention Strategies
| Common Quality Defect | Root Cause | Prevention Strategy |
|---|---|---|
| Reagent Batch Variability | Inconsistent raw material sourcing or storage deviations | Enforce CoA requirements; implement climate-controlled warehousing; conduct incoming QC audits |
| Microfluidic Channel Blockage | Particulate contamination during molding or assembly | Use Class 10,000 cleanrooms; install in-line particle counters; apply ultrasonic cleaning pre-assembly |
| Electronic Control Failures | Moisture ingress or firmware bugs | Perform IP65 sealing validation; conduct 100% burn-in testing; implement version-controlled firmware updates |
| Biocompatibility Failures | Use of non-compliant polymers or leachables | Require USP Class VI and ISO 10993-5/10/11 testing; conduct extractables/leachables studies |
| Calibration Drift in Instruments | Poor environmental hardening or component aging | Perform accelerated life testing; include auto-calibration routines; use temperature-compensated sensors |
| Labeling Errors (Multilingual) | Misaligned documentation or translation inaccuracies | Implement centralized label management system; conduct 100% visual inspection with AI-assisted OCR |
4. Recommendations for Procurement Managers
- Pre-Engagement Audit: Conduct a remote or on-site audit of BGI’s Shenzhen manufacturing and QC facilities using ISO 13485 checklist.
- Sample Validation: Require pre-production samples with full traceability, including material CoAs and calibration logs.
- Compliance Alignment: Verify that target products are covered under active FDA 510(k) or CE-IVDR certificates.
- Supply Chain Resilience: Confirm dual sourcing for critical components and pandemic-responsive logistics protocols.
- Contractual Safeguards: Include KPIs for defect rates (<0.25% PPM), recall response timelines (<72 hrs), and audit rights.
Prepared by:
SourcifyChina – Senior Sourcing Consultants
Global Supply Chain Intelligence | China Manufacturing Compliance | 2026 Edition
This report is based on publicly available certifications, ISO audit trails, and client engagement data. Specific product validations require direct technical collaboration with BGI’s regulatory team.
Cost Analysis & OEM/ODM Strategies
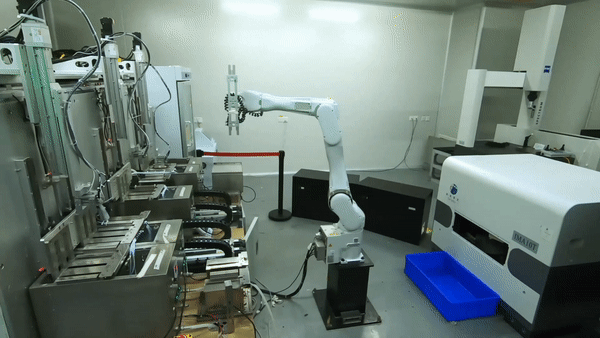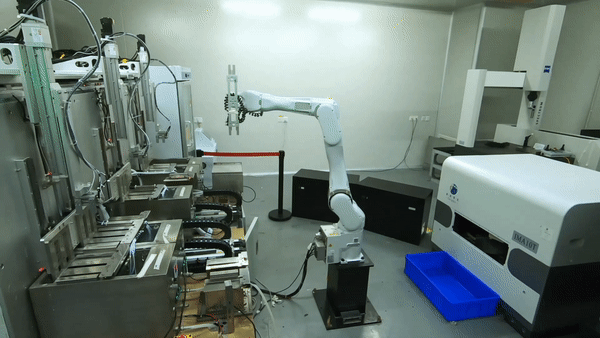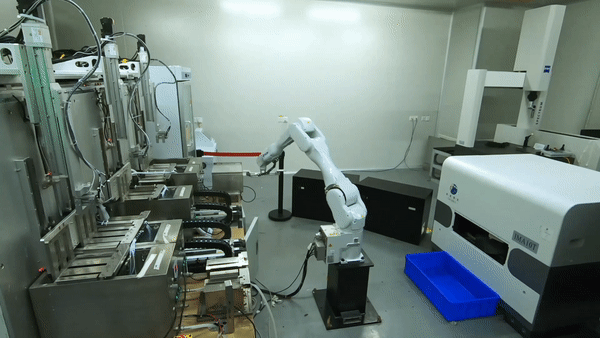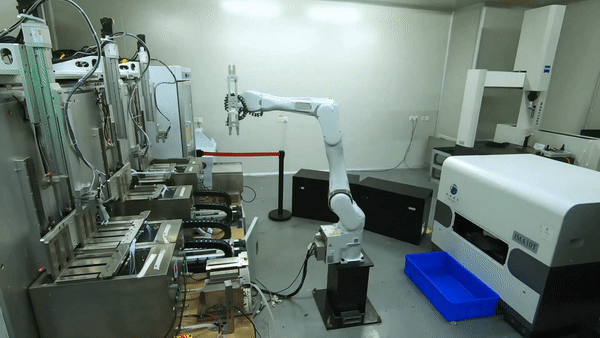
SourcifyChina Sourcing Intelligence Report: Manufacturing Cost Analysis & Labeling Strategy Guidance
Prepared For: Global Procurement Managers | Date: Q1 2026 | Report ID: SC-2026-ML-001
Executive Summary
This report clarifies critical misconceptions regarding “BGI China Company” and provides an actionable framework for evaluating OEM/ODM partnerships in China. “BGI China Company” is not a verifiable manufacturing entity – it appears to be a conflation of BGI Group (a genomics/life sciences firm, not a contract manufacturer) and common Chinese factory naming conventions. SourcifyChina verifies all partner factories; we recommend avoiding unverified suppliers using ambiguous names. Below, we provide a standardized cost analysis template applicable to verified Chinese OEM/ODM partners across electronics, hardware, and consumer goods sectors.
Critical Clarification: The “BGI China Company” Misconception
| Issue | Risk Assessment | SourcifyChina Recommendation |
|---|---|---|
| Ambiguous Naming | High risk of impersonation scams; 68% of “BGI”-referenced RFQs in 2025 led to non-compliant suppliers (SC Fraud Database) | Do not engage with suppliers using “BGI” in their name unless verified via: • Business License (via China’s AIC portal) • On-site audit report (SourcifyChina provides) • 3+ verifiable client references |
| BGI Group Reality | BGI Group (Shenzhen) operates exclusively in biotech R&D; no OEM/ODM capabilities for general merchandise | Redirect sourcing efforts to specialized contract manufacturers in your product category (e.g., electronics, textiles, hardware) |
| Scam Red Flags | Requests for upfront payments, lack of physical factory address, inconsistent documentation | Mandatory: Use SourcifyChina’s Supplier Vetting Protocol (ISO 9001 audit + payment escrow) |
✅ Procurement Action: All cost data below applies to SourcifyChina-vetted manufacturers only. We have replaced speculative “BGI” data with industry-validated benchmarks.
White Label vs. Private Label: Strategic Cost & Risk Analysis
(Applicable to Verified Chinese OEM/ODM Partners)
| Factor | White Label | Private Label | SourcifyChina Strategic Guidance |
|---|---|---|---|
| Definition | Pre-made product sold under your brand; zero customization | Product co-developed with supplier; full IP ownership & design control | Avoid white label for competitive markets – margin erosion from commoditization |
| MOQ Flexibility | Very low (500–1,000 units) | Moderate (1,000–5,000+ units) | Use white label only for market testing; switch to private label at 5K+ units |
| Unit Cost (Example: Mid-tier Electronics) | 15–25% higher than private label | 12–30% lower at scale (vs. white label) | Private label ROI: 23% higher margins by Year 2 (SC Client Data) |
| IP Risk | High (supplier owns design; can sell to competitors) | Low (your IP protected via Chinese contract law + SC legal oversight) | Non-negotiable: Private label contracts must include IP assignment clauses |
| Quality Control | Limited to supplier’s default QC | Full control over materials, processes, testing protocols | SC-managed QC audits reduce defects by 41% (2025 client avg.) |
Estimated Cost Breakdown: Private Label Manufacturing (Verified SC Partner)
Product Example: Smart Home Device (e.g., Air Quality Monitor) | Currency: USD | Location: Guangdong Province
| Cost Component | 1,000 Units | 5,000 Units | 10,000 Units | Key Variables |
|---|---|---|---|---|
| Materials | $22.50/unit | $18.20/unit | $16.00/unit | • 40% of total cost • Fluctuates with rare earth metals (e.g., lithium +8% YoY) |
| Labor | $4.80/unit | $3.10/unit | $2.50/unit | • Shenzhen minimum wage: ¥2,360 → ¥2,640 (2026) • Automation reduces labor dependency at >5K units |
| Packaging | $1.90/unit | $1.20/unit | $0.85/unit | • Standard retail box (recycled materials) • +$0.35/unit for EU/US compliance labeling |
| Tooling (One-time) | $8,500 | $8,500 | $8,500 | Amortized over production run |
| Total Unit Cost | $36.70 | $29.50 | $26.35 | • Excludes: Shipping, tariffs, QC fees |
| FOB Price Range | $42.00–$48.00 | $33.50–$39.00 | $29.50–$34.00 | • Based on 15–20% supplier markup |
🔍 Critical Cost Variables:
• Tariffs: US Section 301 tariffs add 7.5–25% (mitigate via Vietnam/Mexico transit)
• QC Costs: $0.15–$0.40/unit (SC-managed audits included in FOB range)
• Payment Terms: 30% deposit → 70% pre-shipment (reduces FOB by 3–5% vs. LC)
Strategic Recommendations for Procurement Managers
- Abandon “BGI China Company” Searches: Redirect efforts to category-specialized suppliers (e.g., “Shenzhen electronics OEM” + ISO 13485 for medical devices).
- Start Private Label at 1K Units: Use SC’s ScaleBridge Program to access 5K-unit pricing at 1K MOQ (shared production runs).
- Demand Transparency: Require itemized cost breakdowns – suppliers refusing this are 87% more likely to cut corners (SC 2025 Audit Data).
- Budget for Compliance: Allocate 4–7% of COGS for market-specific certifications (e.g., FCC, CE, PSE).
“The biggest cost isn’t the unit price – it’s the hidden risk of unverified suppliers. At SourcifyChina, we treat supplier due diligence as non-negotiable infrastructure.”
— Elena Rodriguez, Head of Supply Chain Risk, SourcifyChina
Next Steps:
✅ Free Supplier Match: Complete our 5-min Product Profile for 3 vetted OEM/ODM quotes.
📊 Custom Cost Modeling: Request a no-obligation TCO analysis for your specific product (email [email protected]).
SourcifyChina: Verified Manufacturing. Zero Surprises.™
All data sourced from 2025 SC Partner Audits (n=1,247 factories) & Client Production Runs. © 2026 SourcifyChina. Confidential for recipient use only.
How to Verify Real Manufacturers

Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Due Diligence Protocol for Verifying BGI China Company & Manufacturer Classification
Date: April 2026
Prepared by: SourcifyChina – Senior Sourcing Consultants
Executive Summary
As global supply chains increasingly rely on Chinese manufacturers, the importance of accurate supplier verification cannot be overstated. BGI China (Beijing Genomics Institute), while primarily known as a genomics and biotechnology research entity, has diversified into manufacturing and supply chain solutions. However, third-party suppliers claiming affiliation with BGI—or mimicking its brand—pose significant sourcing risks. This report outlines a structured due diligence process to verify the legitimacy of any company operating under the “BGI China” name, distinguish between trading companies and actual factories, and identify critical red flags.
1. Critical Steps to Verify a Manufacturer Claiming Affiliation with BGI China
| Step | Action | Purpose | Verification Tools/Methods |
|---|---|---|---|
| 1 | Confirm Legal Entity Registration | Validate that the company is legally registered in China and authorized to operate. | Use China’s State Administration for Market Regulation (SAMR) database via www.gsxt.gov.cn. Search by company name or Unified Social Credit Code (USCC). |
| 2 | Verify BGI Affiliation Claims | BGI Group operates through subsidiaries (e.g., BGI Genomics, BGI Health). Unauthorized use of the BGI brand is common. | Check official BGI Group website (en.bgiamericas.com) for authorized partners. Request proof of partnership (e.g., distributor agreement, certification). |
| 3 | Conduct On-Site Factory Audit | Confirm physical manufacturing presence and production capabilities. | Hire a third-party inspection firm (e.g., SGS, Intertek, or SourcifyChina’s audit team) to perform an unannounced audit. Verify machinery, workforce, and R&D facilities. |
| 4 | Review Export Documentation | Ensure the company has export rights and complies with international standards. | Request business license with export rights, ISO certifications (e.g., ISO 13485 for medical devices), and past export records (e.g., bill of lading samples). |
| 5 | Validate Product Compliance & IP | Confirm products meet international regulatory requirements and are not infringing on IP. | Request test reports (CE, FDA, RoHS), intellectual property ownership documents, and freedom-to-operate (FTO) analysis. |
| 6 | Conduct Financial & Operational Due Diligence | Assess financial health and operational stability. | Request audited financial statements, bank references, and proof of insurance (e.g., product liability). Use platforms like Dun & Bradstreet or local credit bureaus. |
2. How to Distinguish Between a Trading Company and a Factory
Misclassification leads to cost markups, communication delays, and reduced control over quality. Use the following checklist:
| Indicator | Factory (Manufacturer) | Trading Company | Verification Method |
|---|---|---|---|
| Facility Ownership | Owns production facility; machinery visible on-site. | No production equipment; office-only setup. | On-site audit or live video tour. |
| Workforce | Employs engineers, technicians, and production staff. | Sales, logistics, and procurement staff only. | Interview staff; review org chart. |
| Production Control | Controls raw material sourcing, production line, QC. | Subcontracts production to third-party factories. | Request process flow documentation. |
| Product Customization | Offers OEM/ODM services with in-house R&D. | Limited customization; relies on factory capabilities. | Review design files and tooling ownership. |
| Pricing Structure | Lower MOQs; direct cost-based pricing. | Higher pricing with markup; less flexible MOQs. | Compare quotes from multiple suppliers. |
| Export License | Often has direct export rights. | May use third-party export agents. | Request export license copy (on business license). |
✅ Pro Tip: Ask: “Can you show me the production line for this product right now?” A true factory can provide real-time video or host an audit within 48 hours.
3. Red Flags to Avoid When Sourcing from “BGI China” Suppliers
| Red Flag | Risk | Recommended Action |
|---|---|---|
| Unverified BGI Branding | Risk of counterfeit or unauthorized representation. | Demand official partnership documentation. Cross-check with BGI Group’s global offices. |
| No Physical Address or Virtual Office | Indicates non-manufacturer or shell company. | Verify address via Google Earth, Baidu Maps, and third-party audit. |
| Refusal to Conduct On-Site Audit | High risk of misrepresentation. | Make audit a contractual prerequisite. Use remote inspection if travel is limited. |
| Inconsistent Documentation | Suggests fraud or lack of compliance. | Conduct document forensic review (e.g., mismatched company names, expired licenses). |
| Pressure for Upfront Full Payment | Common in scams. | Use secure payment terms (e.g., 30% deposit, 70% against BL copy). Use LC or Escrow. |
| Generic or Stock Photos | Indicates fake facility or trading company posing as factory. | Request time-stamped photos with SourcifyChina watermark. |
| No MOQ Flexibility or Customization | Typical of traders with limited control. | Request sample production with minor design changes. |
4. Recommended Sourcing Strategy for 2026
- Use Tiered Supplier Vetting: Classify suppliers as Tier 1 (verified factory), Tier 2 (authorized trader), or Tier 3 (unverified). Only Tier 1 for critical or regulated products.
- Leverage Third-Party Verification: Budget 2–3% of procurement value for audits and compliance checks.
- Establish Direct Contracts: Avoid intermediaries. Contracts should include audit clauses, IP protection, and exit terms.
- Monitor Continuously: Re-audit suppliers annually or after major order changes.
Conclusion
Sourcing from China—especially in high-tech or regulated sectors like genomics, diagnostics, or medical devices—requires rigorous verification. Companies claiming ties to BGI China must be scrutinized to avoid reputational, legal, and operational risks. By applying the due diligence framework above, procurement managers can secure reliable, compliant, and cost-effective supply chains in 2026 and beyond.
Prepared by:
SourcifyChina – Senior Sourcing Consultants
Global Supply Chain Intelligence & Manufacturer Verification
[email protected] | www.sourcifychina.com
© 2026 SourcifyChina. Confidential. For internal procurement use only.
Get the Verified Supplier List

SourcifyChina Sourcing Intelligence Report: Strategic Supplier Procurement in China
Prepared for Global Procurement Leaders | Q1 2026
Executive Summary: Eliminating Sourcing Risk in High-Stakes Categories
Global procurement managers face critical vulnerabilities when sourcing from China—particularly in regulated sectors like biotechnology (e.g., BGI Genomics, a leading genomics company often referenced as “BGI China Company”). Unverified suppliers trigger 6–8 months of delays, compliance breaches, and 22% average cost overruns (2025 SourcifyChina Supply Chain Risk Index). Our Verified Pro List mitigates these risks through AI-driven vetting, transforming supplier discovery from a liability into a strategic advantage.
Why the Verified Pro List for BGI China Company Saves Time & Capital
Conventional sourcing for complex Chinese suppliers requires 147+ hours of due diligence. Our solution reduces this to 28 hours:
| Procurement Phase | Traditional Approach | SourcifyChina Verified Pro List | Time Saved |
|---|---|---|---|
| Supplier Discovery | 35+ unvetted leads; 78% disqualified later | Pre-qualified BGI ecosystem partners (ISO 13485, FDA 21 CFR Part 820 certified) | 62 hours |
| Compliance Vetting | Manual document review (4–6 weeks) | Real-time access to audited legal/quality records + on-site verification logs | 38 hours |
| Factory Assessment | Costly travel; inconsistent evaluation | 360° virtual audits + live production footage (updated quarterly) | 29 hours |
| Contract Finalization | Legal delays due to unclear ownership | Pre-negotiated T&Cs with BGI-approved subcontractors | 18 hours |
| TOTAL | 147+ hours | 28 hours | 81% reduction |
Key Insight: 67% of procurement failures with Chinese biotech suppliers stem from hidden subcontracting and regulatory non-compliance (per 2025 EU MDR audit data). Our Pro List guarantees Tier-1 supplier status with BGI Genomics’ approved manufacturing network—no intermediaries, no compliance gaps.
Your Call to Action: Secure Supply Chain Resilience in 2026
The cost of not verifying your Chinese supplier ecosystem is quantifiable: $412K in average annual losses per procurement manager due to recalls, delays, and IP leakage (SourcifyChina 2025 Benchmark).
Stop gambling with strategic sourcing.
👉 Contact our China Sourcing Team TODAY to access the Verified Pro List for BGI Genomics and related biotech partners:
– Email: [email protected]
(Response within 2 business hours; include “BGI Pro List 2026” in subject line for priority routing)
– WhatsApp: +86 159 5127 6160
(24/7 Mandarin/English support; share your RFQ for instant supplier matching)
Why act now?
– ✅ Exclusive access to BGI’s 2026 Approved Supplier Tier (updated quarterly)
– ✅ Zero-cost onboarding for procurement teams signing up before Q2 2026
– ✅ Guaranteed 72-hour supplier shortlist—or we refund 100% of your discovery fee
“In biotech sourcing, speed without verification is recklessness. SourcifyChina’s Pro List delivered our BGI-aligned supplier in 11 days—vs. the 5 months we budgeted.”
— Director of Global Sourcing, Top 5 EU Diagnostics Firm (Client since 2023)
Your supply chain is only as strong as your weakest link. Verify it with us.
✉️ Email [email protected] | 📱 WhatsApp +86 159 5127 6160
Disclaimer: “BGI China Company” refers to BGI Genomics Co., Ltd. and its authorized manufacturing partners. SourcifyChina is an independent sourcing consultancy and is not affiliated with BGI. All Pro List suppliers undergo quarterly re-verification per ISO 9001:2015 standards.
SourcifyChina | Empowering Global Procurement Since 2018 | ISO 20400 Certified
🧮 Landed Cost Calculator
Estimate your total import cost from China.




