The global alginato de sodio (sodium alginate) market is experiencing steady growth, driven by increasing demand across food and beverage, pharmaceutical, and biomedical applications. According to a report by Mordor Intelligence, the global sodium alginate market was valued at approximately USD 470 million in 2023 and is projected to grow at a CAGR of over 5.2% from 2024 to 2029. This expansion is fueled by rising consumer preference for natural additives and hydrocolloids in food stabilization, as well as growing use in wound care and drug delivery systems. North America and Europe remain key markets due to strict regulatory standards favoring clean-label ingredients, while Asia-Pacific is witnessing accelerated adoption in industrial and healthcare sectors. With such momentum, identifying the leading manufacturers becomes critical for supply chain reliability and product innovation. Based on market presence, production capacity, and application reach, the following seven companies stand out as the top sodium alginate producers worldwide.
Top 7 Alginato De Sodio Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Sodium Alginate BP Ph Eur IP FCC Food Grade n …
Domain Est. 2005 | Founded: 1976
Website: mubychem.com
Key Highlights: Supplier, Manufacturer, Exporter of Sodium Alginate BP Ph Eur IP FCC Food Grade, Muby Chemicals of Mubychem Group, established in 1976, is the original ……
#2 Food Grade Sodium Alginate Suppliers
Domain Est. 2019
Website: gumstabilizer.com
Key Highlights: Gino is one of the professional and experienced food grade sodium alginate suppliers in China. Find your best E401 alginate producers in China!…
#3 Sodium Alginate
Domain Est. 1997
Website: pubchem.ncbi.nlm.nih.gov
Key Highlights: Sodium Alginate is the sodium salt form of alginic acid and gum mainly extracted from the cell walls of brown algae, with chelating activity….
#4 Sodium alginate
Domain Est. 2000
Website: quiminet.com
Key Highlights: Sodium alginate: Compra y Venta, Nombres Alternativos, Imágenes, Ofertas, Manejo, Artículos, Noticias, Propiedades….
#5 NovaMatrix Alginate and Hyaluronate Biomaterials
Domain Est. 2002
Website: novamatrix.biz
Key Highlights: NovaMatrix® manufacture PRONOVA® sodium alginate in our facilities in Norway. We operate according to GMP guidelines and ISO standards; ISO 9001:2015 and ISO ……
#6 Alginato de Sodio
Domain Est. 2007
#7 Alginato de Sodio
Domain Est. 2024
Website: hylixchem.com
Key Highlights: Como fabricante y distribuidor confiable de polvo de alginato de sodio, Hylix Chemicals ofrece calidad consistente, excelente solubilidad y rendimiento ……
Expert Sourcing Insights for Alginato De Sodio

H2: Projected Market Trends for Sodium Alginate (Alginato de Sodio) in 2026
Based on current industry dynamics, technological advancements, and macroeconomic factors, the global sodium alginate market is poised for significant evolution by 2026. Key trends shaping the landscape include:
1. Sustained Growth in Food & Beverage Applications:
Demand for clean-label, plant-based, and functional food ingredients will drive sodium alginate usage. Its role as a natural thickener, stabilizer, and gelling agent aligns with consumer preferences for minimally processed foods. Growth is expected in plant-based dairy alternatives (e.g., vegan cheeses, yogurts), gluten-free products, and encapsulated flavors/nutrients. The rise of personalized nutrition and fortified foods will further boost demand.
2. Expansion in Pharmaceutical and Medical Innovations:
Sodium alginate’s biocompatibility and mucoadhesive properties will fuel its use in advanced drug delivery systems, wound healing dressings (especially for chronic wounds), and tissue engineering scaffolds. By 2026, increased R&D in regenerative medicine and targeted therapies is expected to open new high-value applications, particularly in 3D bioprinting and oral sustained-release formulations.
3. Rising Demand in Emerging Biotechnological and Cosmetic Uses:
The personal care industry will increasingly adopt sodium alginate in anti-aging creams, masks, and hydrating formulations due to its film-forming and moisture-retention properties. Simultaneously, its use in encapsulation technologies for probiotics, enzymes, and bioactive compounds in nutraceuticals and agriculture will expand, driven by sustainability and efficacy goals.
4. Supply Chain Resilience and Sustainability Focus:
With seaweed farming concentrated in regions like China, Norway, and Chile, geopolitical and environmental risks (e.g., ocean warming, overharvesting) will push companies toward diversified sourcing and investment in sustainable aquaculture practices. Certification schemes (e.g., MSC, ASC) and traceability technologies (blockchain) will gain importance, influencing procurement decisions.
5. Technological Advancements in Extraction and Modification:
Efforts to improve yield, purity, and functionality will lead to wider adoption of green extraction methods (e.g., enzyme-assisted, ultrasound, microwave) and tailored modification techniques. Customized alginates with specific G/M ratios or molecular weights will meet niche requirements in high-performance applications, enhancing product differentiation.
6. Regulatory Harmonization and Quality Standards:
As global trade expands, harmonization of regulatory standards (e.g., FDA, EFSA, FSSAI) for sodium alginate in food and medical devices will become critical. Manufacturers investing in compliance and quality assurance (GMP, ISO) will gain competitive advantage, especially in regulated markets.
7. Price Volatility and Strategic Sourcing:
Fluctuations in raw seaweed supply due to climate variability and seasonal harvesting may lead to price instability. Long-term contracts, vertical integration (e.g., owning seaweed farms), and regional production hubs are expected strategies to mitigate risk and secure supply.
In summary, by 2026, the sodium alginate market will be characterized by innovation-driven growth, expanding into high-tech applications while facing challenges related to sustainability and supply security. Companies that prioritize R&D, sustainable sourcing, and regulatory compliance are likely to lead the market.

Common Pitfalls Sourcing Alginato De Sodio (Quality, IP)
Sourcing high-quality sodium alginate (Alginato de Sodio) for pharmaceutical or food applications requires careful attention to avoid common pitfalls, particularly concerning quality and intellectual property (IP) compliance. Below are key challenges to be aware of:
Inadequate Quality Control and Specification Verification
One of the most frequent issues when sourcing sodium alginate is receiving material that does not meet required quality standards. Suppliers, especially from low-cost regions, may provide certificates of analysis (CoA) that appear compliant but lack third-party verification. Critical parameters such as viscosity, gel strength, heavy metal content (e.g., lead, arsenic), microbial load, and degree of esterification must be rigorously tested. Failure to verify these specifications can lead to product inconsistency, failed batch releases, or regulatory non-compliance.
Misrepresentation of Grade (Pharmaceutical vs. Industrial)
Sodium alginate is available in industrial, food, and pharmaceutical grades. A common pitfall is suppliers marketing industrial-grade material as pharmaceutical-grade (e.g., complying with USP/NF, Ph. Eur., or JP standards) without proper certification. Buyers must confirm that the material is manufactured under Good Manufacturing Practices (GMP), comes with a Drug Master File (DMF), and includes full traceability of raw materials (e.g., seaweed source and processing methods).
Lack of Intellectual Property (IP) and Regulatory Documentation
When sourcing sodium alginate for pharmaceutical formulations, especially in regulated markets, it’s essential to ensure the supplier has clear IP rights and provides necessary regulatory support. Pitfalls include:
– Uncleared patents: Using sodium alginate in specific delivery systems (e.g., gastroretentive tablets or wound dressings) may be covered by third-party patents. Sourcing from a supplier without freedom-to-operate (FTO) analysis can expose the buyer to IP litigation.
– Incomplete documentation: Missing or incomplete regulatory dossiers (e.g., CEP, ASMF) can delay product registration and market entry.
Supply Chain Transparency and Sustainability Concerns
The source of raw seaweed (typically Macrocystis pyrifera or Ascophyllum nodosum) impacts alginate quality and consistency. Opaque supply chains may hide unsustainable harvesting practices or contamination risks (e.g., from heavy metals or microplastics). Buyers should require evidence of sustainable sourcing, environmental certifications, and batch-to-batch consistency.
Poor Supplier Qualification and Audit Practices
Relying solely on price without conducting on-site audits or reviewing supplier quality systems increases the risk of receiving substandard material. Qualified suppliers should have robust quality management systems (e.g., ISO 13485 for medical devices or ISO 22000 for food), and be open to audits.
Inconsistent Batch-to-Batch Performance
Due to natural variation in seaweed, sodium alginate properties can vary significantly between batches. Suppliers that do not perform blending or standardization may deliver inconsistent viscosity or gelling behavior, affecting product performance—especially in sensitive applications like 3D bioprinting or controlled drug release.
Avoiding these pitfalls requires due diligence: vetting suppliers thoroughly, demanding complete and verifiable documentation, and establishing long-term quality agreements with clear specifications and accountability.
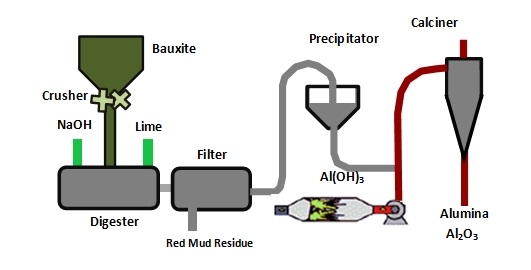
Logistics & Compliance Guide for Alginato De Sodio (Sodium Alginate)
Overview of Alginato De Sodio
Alginato de Sodio, also known as Sodium Alginate, is a natural polysaccharide extracted from brown seaweed. It is commonly used in the food, pharmaceutical, and cosmetic industries as a gelling agent, thickener, and stabilizer. Due to its widespread applications, adherence to proper logistics and regulatory compliance is essential during import, export, storage, and handling.
Chemical and Physical Properties
- Chemical Formula: (C₆H₇O₆Na)ₙ
- Appearance: White to yellowish fibrous powder or granules
- Solubility: Soluble in water, insoluble in organic solvents
- Stability: Stable under normal conditions; sensitive to strong acids and heavy metal ions
- CAS Number: 9005-38-3
Understanding these properties is crucial for safe handling and transportation.
Regulatory Classification
Alginato de Sodio is generally recognized as safe (GRAS) by the U.S. FDA and approved as a food additive (E401) in the European Union. Regulatory status may vary by country:
– Food Use: Approved globally under food additive regulations (e.g., EU Regulation (EC) No 1333/2008)
– Pharmaceutical Use: Complies with USP, Ph. Eur., or JP standards if used in medicinal products
– Cosmetics: Approved by INCI and subject to regional cosmetic regulations
Ensure compliance with target market regulations prior to shipment.
Packaging Requirements
- Use moisture-resistant, sealed packaging (e.g., multi-wall paper bags with polyethylene lining or HDPE drums)
- Label packages clearly with:
- Product name: “Alginato de Sodio” or “Sodium Alginate”
- CAS Number: 9005-38-3
- Batch/lot number
- Net weight
- Manufacturer and supplier information
- Applicable hazard symbols (if any)
Avoid contamination by storing away from strong acids, heavy metals, and foodstuffs not intended for industrial use.
Storage Conditions
- Temperature: Store in a cool, dry place below 30°C (86°F)
- Humidity: Maintain low humidity to prevent caking or degradation
- Shelf Life: Typically 2–3 years when stored properly
- Segregation: Keep separate from incompatible materials such as strong acids, oxidizing agents, and heavy metal salts
Monitor storage conditions regularly to maintain product quality.
Transportation Guidelines
- Mode of Transport: Suitable for road, sea, and air freight
- UN Number: Not classified as hazardous under UN transport regulations (e.g., IMDG, IATA, ADR)
- Documentation: Include safety data sheet (SDS), certificate of analysis (COA), and commercial invoice
- Labeling: No hazardous labels required for transportation unless contaminated
Confirm carrier-specific requirements and ensure proper ventilation during transit.
Safety Data Sheet (SDS) Compliance
An up-to-date SDS in the local language (e.g., Spanish for “Alginato de Sodio”) must accompany shipments. Key sections include:
– Hazard identification (typically non-hazardous)
– First-aid measures
– Fire-fighting measures
– Accidental release measures
– Handling and storage
– Exposure controls and personal protection
Review SDS before use and train personnel accordingly.
Import/Export Documentation
Ensure all shipments include:
– Commercial invoice
– Packing list
– Certificate of Analysis (COA)
– Certificate of Free Sale (if required)
– Phytosanitary certificate (if applicable, depending on country of origin)
– SDS in destination country language
Check import regulations in the destination country, especially for use in food or pharmaceuticals.
Customs Classification (HS Code)
The Harmonized System (HS) code for Sodium Alginate varies by region:
– United States: 3913.10 (Alginic acid, its salts and esters)
– European Union: 3913.10.00
– Mexico: 3913.10.01
– Other Countries: Verify with local customs authorities
Accurate classification ensures correct tariffs and regulatory compliance.
Environmental and Disposal Considerations
- Biodegradability: Sodium alginate is biodegradable
- Disposal: Dispose of according to local waste regulations. Can typically be disposed of as non-hazardous waste
- Spills: Collect dry material and place in a suitable container. Avoid creating dust
Follow local environmental protection guidelines.
Quality Assurance and Traceability
- Maintain batch traceability from raw material to final product
- Conduct regular quality testing (viscosity, purity, microbiological content)
- Use certified suppliers adhering to GMP, ISO 9001, or FSSC 22000 standards
Ensure documentation supports quality claims for audits and regulatory inspections.
Conclusion
Proper logistics and compliance management for Alginato de Sodio are essential to ensure product safety, regulatory adherence, and supply chain efficiency. Always verify regional regulations, use correct documentation, and follow safe handling practices throughout the distribution process.
Conclusión sobre el abastecimiento de alginato de sodio
La selección y el abastecimiento de alginato de sodio requieren una evaluación cuidadosa de varios factores clave, tales como la calidad del producto, el cumplimiento de normas internacionales (como USP, Ph. Eur., o FAO/WHO), la trazabilidad de la materia prima, la estabilidad del suministro y la relación costo-beneficio. Este polisacárido, derivado de algas marinas, es ampliamente utilizado en industrias farmacéutica, alimentaria y biomédica debido a sus propiedades gelificantes, estabilizantes y de encapsulación.
En conclusión, para garantizar un suministro eficiente y sostenible de alginato de sodio, es fundamental establecer alianzas con proveedores confiables y certificados, preferiblemente con capacidad de escalabilidad y enfoque en sostenibilidad. Además, se recomienda implementar un proceso de evaluación continua del desempeño del proveedor y mantener un monitoreo constante de las variaciones en la calidad del producto. Una estrategia de sourcing bien estructurada no solo asegura la calidad del alginato de sodio, sino que también contribuye a la eficiencia operativa y al cumplimiento regulatorio en las aplicaciones finales.