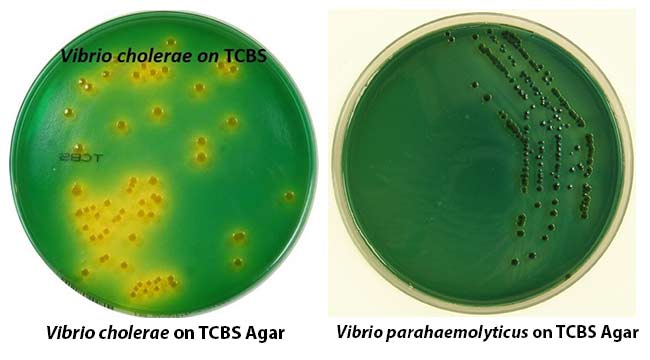
The global agar TCBS (Thiosulfate-Citrate-Bile Salts-Sucrose) media market is experiencing steady growth, driven by rising demand for microbiological testing in food safety, pharmaceuticals, and clinical diagnostics. According to Mordor Intelligence, the culture media market — which includes selective media like agar TCBS — is projected to grow at a CAGR of over 7.5% from 2023 to 2028, with Asia-Pacific emerging as a key growth region due to increasing regulatory scrutiny and food export requirements. Agar TCBS, specifically used for the isolation of Vibrio species such as V. cholerae and V. parahaemolyticus, plays a critical role in public health and seafood safety monitoring. This growing need for reliable pathogen detection has spurred expansion among manufacturers specializing in high-quality, consistent agar formulations. Based on production capacity, global distribution, regulatory compliance (including ISO and FDA standards), and product innovation, the following seven manufacturers have established themselves as leaders in the agar TCBS space.
Top 7 Agar Tcbs Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 BD DIFCO™ TCBS Agar 500g
Domain Est. 1990
Website: bd.com
Key Highlights: BD supplies more than 400 different BD Difco™ and BD BBL™ brand media formulations and ingredients in dehydrated form for the convenience of the ……
#2 CRITERION™ TCBS (Thiosulfate Citrate Bile Salts Sucrose) Agar …
Domain Est. 1996
Website: hardydiagnostics.com
Key Highlights: Hardy Diagnostics CRITERION™ TCBS (Thiosulfate Citrate Bile Salts Sucrose) Agar is recommended for the selective isolation and cultivation of Vibrio spp….
#3 Tcbs agar
Domain Est. 1998
Website: sigmaaldrich.com
Key Highlights: Find tcbs agar and related products for scientific research at MilliporeSigma….
#4 Neogen® Thiosulfate Citrate Bile Salts Sucrose (TCBS) Agar
Domain Est. 1999
Website: neogen.com
Key Highlights: Thiosulfate Citrate Bile Salts Sucrose (TCBS) Agar is used for the selective isolation of Vibrio cholerae and other enteropathogenic vibrios….
#5 TCBS Agar
Domain Est. 2000
#6 TCBS Agar for Vibrio Species: Principles and Practices
Domain Est. 2015
Website: tmmedia.in
Key Highlights: TCBS Agar is used to isolate and identify Vibrio species like V. cholerae and V. parahaemolyticus in food, water, and clinical samples….
#7 THIOSULFATE CITRATE BILE SACCHAROSE AGAR
Domain Est. 2015
Website: alliance-bio-expertise.com
Key Highlights: Solid medium for the selective isolation of Vibrio spp, and Vibrio parahaemolyticus according to the ISO standard. Prices on request or available for ……
Expert Sourcing Insights for Agar Tcbs

H2: Market Trends for Agar TCBS in 2026
As we approach 2026, the global market for Thiosulfate Citrate Bile Salts Sucrose (TCBS) Agar—a selective culture medium primarily used for isolating and differentiating Vibrio species such as V. cholerae and V. parahaemolyticus—is expected to experience notable shifts driven by public health priorities, technological advancements, and evolving regulatory landscapes. Below is an analysis of the key market trends shaping the Agar TCBS sector in 2026:
-
Increased Demand in Food and Water Safety Testing
With rising global concerns over foodborne and waterborne illnesses, especially in emerging economies, regulatory bodies are tightening safety standards. TCBS Agar remains a gold standard in detecting pathogenic Vibrio species in seafood, coastal water, and clinical samples. The expansion of aquaculture and seafood exports—particularly from Southeast Asia and Latin America—is fueling demand for reliable microbiological testing, boosting the use of TCBS Agar in quality control laboratories. -
Growth in Clinical Diagnostics in Developing Regions
In regions with high incidence of cholera and other Vibrio-related infections—such as Sub-Saharan Africa, South Asia, and parts of Latin America—healthcare infrastructure improvements are increasing access to diagnostic microbiology. International aid programs and public health initiatives (e.g., WHO’s Global Task Force on Cholera Control) are supporting laboratory capacity building, leading to higher adoption of cost-effective, reliable media like TCBS Agar. -
Technological Enhancements and Product Innovation
Leading manufacturers are investing in ready-to-use formulations, dehydrated powder improvements, and chromogenic variants of TCBS Agar to reduce preparation time and increase specificity. By 2026, expect wider adoption of pre-poured plates and automated inoculation-compatible formats, especially in high-throughput diagnostic and research labs. Innovations in shelf-life stability and reduced contamination risk are also enhancing product appeal. -
Consolidation and Competition Among Suppliers
The market is witnessing consolidation among major life science and microbiology media suppliers (e.g., Thermo Fisher Scientific, bioMérieux, Merck KGaA, and HiMedia Laboratories). This is leading to competitive pricing, expanded distribution networks, and bundled diagnostic solutions. Regional players, particularly in India and China, are gaining market share by offering cost-effective alternatives without compromising quality. -
Regulatory and Standardization Pressures
Regulatory agencies such as the FDA, EU-EMA, and Codex Alimentarius are emphasizing standardized microbiological methods in food and clinical testing. TCBS Agar is included in international guidelines (e.g., ISO/TS 21872 for Vibrio detection), reinforcing its role as a benchmark medium. Compliance with ISO 13485 and GMP standards is becoming a differentiator for manufacturers. -
Impact of Climate Change and Infectious Disease Surveillance
Climate change is expanding the geographic range of Vibrio species due to warmer sea temperatures and extreme weather events. This ecological shift is increasing the need for active surveillance in non-endemic areas, prompting public health labs in North America and Europe to integrate TCBS Agar into routine monitoring programs, especially post-flooding or coastal contamination events. -
Sustainability and Environmental Concerns
There is growing scrutiny on the environmental impact of single-use plastic cultureware and chemical waste from microbiological testing. By 2026, manufacturers are responding with eco-friendly packaging, reduced chemical load formulations, and recycling programs—though the core composition of TCBS Agar remains largely unchanged due to its proven efficacy.
Conclusion:
The Agar TCBS market in 2026 is poised for steady growth, driven by persistent public health needs, regulatory enforcement, and technological refinement. While molecular methods (e.g., PCR, NGS) are gaining ground, TCBS Agar retains a critical role due to its affordability, simplicity, and reliability—especially in resource-limited settings. Stakeholders should focus on scalability, quality assurance, and integration with digital lab systems to remain competitive.

Common Pitfalls Sourcing Agar TCBS (Quality and Intellectual Property)
Sourcing Thiosulfate-Citrate-Bile Salts-Sucrose (TCBS) Agar, a selective and differential medium used primarily for isolating Vibrio species, involves several critical considerations. Overlooking quality and intellectual property (IP) aspects can lead to unreliable test results, regulatory non-compliance, or legal risks. Below are key pitfalls to avoid:
Quality-Related Pitfalls
Inconsistent Batch-to-Batch Performance
One of the most common quality issues is variability between production batches. Poor manufacturing controls can lead to differences in pH, osmolarity, or nutrient composition, affecting the selective and differential properties of TCBS Agar. This inconsistency may result in false negatives or reduced recovery of target Vibrio species.
Substandard Raw Materials
The performance of TCBS Agar heavily depends on the quality of its components—particularly bile salts, citrate, and sucrose. Sourcing from suppliers using low-grade or impure raw materials can compromise selectivity, allowing non-target organisms to grow or inhibiting Vibrio growth.
Improper Formulation or Preparation
Some suppliers may alter the original TCBS formulation to reduce costs, potentially affecting diagnostic accuracy. Additionally, improper drying, granulation, or hydration instructions can impact the medium’s performance. Always verify that the formulation adheres to recognized standards (e.g., ISO, FDA, or Harmonized Methods).
Lack of Performance Testing and Quality Certifications
Reputable suppliers provide lot-specific certificates of analysis (CoA), including growth promotion tests using reference Vibrio strains (e.g., V. cholerae, V. parahaemolyticus) and inhibition tests for non-target organisms. Sourcing from suppliers without these validations increases the risk of using ineffective media.
Inadequate Storage and Shelf-Life Management
TCBS Agar is sensitive to moisture, light, and temperature. Poor storage conditions during shipping or at the supplier level can degrade components like bile salts or indicators, reducing shelf life and performance. Always confirm the supplier’s storage and shipping protocols.
Intellectual Property (IP) Pitfalls
Use of Copycat or Unlicensed Formulations
The original TCBS Agar formulation is well-established and may be protected under patents or trademarks held by original developers or major diagnostic companies. Sourcing from manufacturers who reproduce branded formulations without proper licensing can expose users to IP infringement risks, especially in regulated or commercial testing environments.
Misleading Branding and Trademark Violations
Some suppliers may use names or packaging that closely resemble trademarked products (e.g., “TCBS-like” or similar color schemes), creating confusion and potential legal exposure. Always verify that product names and branding do not infringe on existing trademarks.
Lack of Transparency in Manufacturing Origin
Suppliers may not disclose where the agar is manufactured or whether they have rights to produce the formulation. Sourcing from opaque supply chains increases the risk of inadvertently using counterfeit or IP-violating products, particularly with offshore manufacturers.
Absence of Regulatory Compliance Documentation
In regulated industries (e.g., food safety, clinical diagnostics), using media with unclear IP status or lacking regulatory filings (such as FDA Master Files or CE declarations) can impede audits or product approvals. Ensure suppliers can provide full documentation supporting both quality and legal standing.
Recommendations to Mitigate Risks
- Source TCBS Agar from reputable, established manufacturers with transparent quality control processes.
- Request and review Certificates of Analysis and growth performance data for each lot.
- Confirm that the formulation complies with international standards (e.g., ISO 21872).
- Verify trademark and patent status, especially when using branded equivalents.
- Establish supplier qualifications audits, including site visits or quality agreements.
By carefully evaluating both quality assurance practices and intellectual property compliance, laboratories can ensure reliable microbiological results and avoid legal or operational setbacks when sourcing TCBS Agar.
Logistics & Compliance Guide for Agar TCBS
Overview
Thiosulfate Citrate Bile Salts Sucrose (TCBS) Agar is a selective and differential culture medium used primarily for the isolation and identification of Vibrio species, particularly Vibrio cholerae and Vibrio parahaemolyticus, from clinical and environmental samples. Proper logistics and compliance handling ensures the medium remains effective and safe throughout its lifecycle.
Storage Requirements
TCBS Agar should be stored according to manufacturer specifications to maintain integrity. Typically:
– Store in a cool, dry place between 15°C and 25°C.
– Protect from light and moisture.
– Keep containers tightly sealed to prevent clumping or degradation.
– Avoid freezing, as it may affect medium performance.
Transportation Guidelines
- Ship in sealed, labeled containers with desiccants to prevent moisture absorption.
- Use temperature-controlled transport if ambient conditions exceed recommended storage ranges.
- Ensure packaging complies with international shipping regulations for diagnostic preparations (e.g., IATA/IMDG, as applicable).
- Label packages with appropriate hazard and handling information (non-hazardous for transport if classified as such).
Regulatory Compliance
- Classification: TCBS Agar is generally classified as a non-hazardous biological preparation for transport (UN3373, Biological Substance, Category B, if containing inoculated cultures; otherwise, typically exempt).
- Labeling: Follow local and international labeling standards. Include product name, batch/lot number, expiry date, storage conditions, and manufacturer details.
- Documentation: Maintain Certificates of Analysis (CoA), Safety Data Sheets (SDS), and regulatory compliance documentation (e.g., ISO 13485, if applicable).
- Import/Export: Verify country-specific requirements for diagnostic microbiological media. Some regions may require permits or customs declarations.
Handling and Usage
- Use personal protective equipment (PPE) such as gloves and lab coats when handling.
- Reconstitute and prepare according to manufacturer instructions under sterile conditions.
- Decontaminate used plates and materials via autoclaving (121°C, 15 psi, 30–60 minutes) before disposal.
- Follow biosafety level (BSL-2) practices when culturing pathogenic Vibrio species.
Expiry and Quality Control
- Do not use beyond the printed expiration date.
- Perform growth promotion testing with control strains (e.g., Vibrio cholerae ATCC® 14035™ and Vibrio parahaemolyticus ATCC® 17802™) upon receipt and periodically during use.
- Monitor for contamination, moisture, or discoloration before use.
Disposal Procedures
- Treat used TCBS Agar plates and cultures as biohazardous waste.
- Dispose of in accordance with local, national, and international biohazard waste regulations (e.g., WHO, CDC, or EPA guidelines).
- Use authorized medical/biological waste disposal services where required.
Record Keeping
Maintain logs for:
– Inventory tracking (receipt, usage, disposal).
– Temperature monitoring for storage areas.
– Quality control results.
– Training records for personnel handling the medium.
Adherence to this guide ensures regulatory compliance, product efficacy, and user safety in clinical, research, and public health laboratory settings.
Conclusion for Sourcing Agar TCBS:
In conclusion, sourcing TCBS (Thiosulfate-Citrate-Bile Salts-Sucrose) agar requires careful consideration of quality, supplier reliability, regulatory compliance, and cost-effectiveness. As a selective and differential medium essential for the isolation and identification of Vibrio species, particularly in clinical, food, and environmental testing, the performance and consistency of TCBS agar are critical. It is imperative to select reputable suppliers with strong quality control measures, proper documentation (such as Certificate of Analysis and ISO certification), and the ability to provide stable, contamination-free products with batch-to-batch consistency.
Additionally, factors such as shelf life, storage conditions, and technical support should be evaluated to ensure optimal functionality in laboratory workflows. Establishing long-term partnerships with trusted vendors can enhance supply chain reliability and ensure uninterrupted operations, especially in diagnostic and regulatory settings. Ultimately, a strategic sourcing approach for TCBS agar balances quality assurance with cost efficiency, supporting accurate and reproducible microbiological results.